Your Location:Home >Products >OLED intermediates >Boric acids >126747-14-6

Product Details
Description
4-Cyanophenylboronic acid can be used for palladium catalyzed Suzuki cross-coupling reactions to synthesize 6-acryl-2,4-diamino-pyrimidines and triazines. It is used as precursor in the synthesis of inhibitors such as Tpl2 kinase inhibitors and P2X7 antagonists used in the treatment of pain.?It is also a reagent used for preparation of himbacine analogs as thrombin receptor antagonists and potential antiplatelet agents, trisulfonated calixarene upper-rim sulfonamido and their complexation with trimethyllysine epigenetic mark, antimalarial compounds via Suzuki cross-coupling, and deoxyuridine derivatives.
Reference
G. Cooke, H. A. de Cremiers, V. M. Rotello, B. Tarbit, P. E. Vanderstr?ten, Synthesis of 6-aryl-2,4-diamino-pyrimidines and triazines using palladium catalysed Suzuki cross-coupling reactions, Tetrahedron, 2001, vol. 57, pp. 2787-2789 N. Ni, H. Chou, J. Wang, M. Li, C. Lu, P. C. Tai, B. Wang, Identification of boronic acids as antagonists of bacterial quorum sensing in Vibrio harveyi, Biochemical and Biophysical Research Communications, 2008, vol. 369, pp. 590-594
Chemical Properties
white to yellow powder
Uses
suzuki reaction
Uses
4-Cyanophenylboronic Acid is a reactant used in the synthesis of (AMG-8718), an inhibitor of β-site amyloid precursor protein cleaving enzyme (BACE1).
Uses
Intermediates of Liquid Crystals
InChI:InChI=1/C7H6BNO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4,10-11H
Nine novel terphenyl-bridged bisbenzimidazoles were synthesized in high yield by the condensation of o-phenylenediamines and terphenyl dinitriles in the presence of polyphosphoric acid. The bisbenzimidazoles are thermally robust with high decomposition te
The Alcaraz-Vaultier borylation of aryl halides and triflates is reported utilizing diisopropylaminoborane (BH2N(iPr)2) prepared from the corresponding lithium aminoborohydride (LAB reagent). BH 2N(iPr)2, prepared by reacting lithium diisopropylaminoborohydride with trimethylsilyl chloride, provided the most consistent isolated yields from this reaction. Catalytic amounts of palladium dichloride produced the highest yields from aryl iodides, while catalytic tris(dibenzylideneacetone)dipalladium(chloroform) provided the best yields for aryl bromides and triflates. This route to boronic acids is mild enough to tolerate various functionalities and for the first time employs aryl triflates as substrates for the Alcaraz-Vaultier borylation. In addition, it was found that both boronic acid and ester compounds could be isolated from the reaction mixture utilizing simple work-up procedures. Treatment of the reaction intermediate with an acid/base work-up provided the corresponding boronic acid, while treating the same intermediate with a diol, such as neopentyl glycol, afforded the corresponding boronic ester.
Triarylamine-based dual-function coadsorbents containing a carboxylic acid acceptor linked by extended π-conjugation aryl linkers (e.g., phenylene: HC-A3, naphthalene: HC-A4 and anthracene: HC-A5) were newly designed and synthesized. They were used as coadsorbents in organic dye-sensitized solar cells (DSSCs) based on a porphyrin dye (hexyloxy-biphenyl-ZnP-CN-COOH (HOP)). For comparison, the π-conjugated phenyl linker (HC-A3) previously developed by our group was also used as a coadsorbent. The structural effects on the photophysical and electrochemical properties and DSSC performance were systematically investigated. As a result, the DSSCs based on HC-A4 and HC-5 displayed power conversion efficiencies (PCEs) of 8.2% and 5.1%, respectively, while the HC-A3-based DSSC achieved a PCE of 7.7%. In the case of HC-A4, both the short-circuit photocurrent densities (Jsc) and open-circuit voltages (Voc) of DSSCs were simultaneously improved to a large extent due to the more effective prevention of π-π stacking of organic dye molecules and the better light-harvesting effect at short wavelengths. The HC-A5-based DSSC exhibited a much lower short-circuit current (Jsc) and open-circuit voltages (Voc) compared to the HC-A4-based DSSC, due to the fact that the dihedral angle of the π-conjugated linkers was too high for electron injection into the TiO2 conduction band (CB) level. This had a reduced effect on preventing the π-π stacking of dye molecules, resulting in lower Jsc and Voc values.
A general and convenient protocol for the electrophilic borylation of aryl Grignard reagents prepared from arylbromides by direct insertion of magnesium in the presence of LiCl or by Mg/Br exchange with iPrMgCl?LiCl has been developed. Various aryl boronic acids were synthesized in a straightforward manner in excellent yields at 0 °C.
Novel disubstituted propiolates bearing chromophoric terphenylene mesogenic groups, namely, 40-cyano- 4-terphenylyl-2-octynate M(CN) and 40-methoxyl-4-terphenylyl- 2-octynate M(OCH3) are synthesized, where the terphenyl groups are connected to the C≡C through ester linkage directly. Using transition-metal catalysts such as the classical MoCl 5- and WCl6-based metathesis catalysts, the polymerization of the M(CN) and M(OCH3) are carried out in a series of different solution, however, did not obtain any products. It suggests that the WCl 6- and MoCl5-based catalysts are poisoned by the polar groups, on the other hand, the bulk terphenyl groups and the long alkyl chain around the C≡C bond might inhibit the reaction. M(CN) displays monotropic nematicity, whereas M(OCH3) exhibits enantiotropic nematicity and smecticity (SmAd) with a bilayer arrangement when cooled and heated. Ultraviolet spectroscopy and photoluminescence measurements also show that the terphenyl groups endow disubstituted propiolates with strong UV light absorption and high photoluminescence. Akademiai Kiado, Budapest, Hungary 2009.
In this study, we developed a simple transition-metal-free borylation reaction of aryl bromides. Bis-boronic acid (BBA), was used, and the borylation reaction was performed using a simple procedure at a mild temperature. Under mild conditions, aryl bromides were converted to arylboronic acids directly without any deprotection steps and purified by conversion to trifluoroborate salts. The functional group tolerance was considerably high. The mechanism study suggested that this borylation reaction proceeds via a radical pathway.
The invention relates to a preparation method of four nimesulide derivatives with the function of treating liver malignant tumors. According to the invention, a nimesulide solution and an acid or alkaline substance are subjected to displacement reaction to generate three salts with better water solubility and more stability, which are the nimesulide derivatives, or p-aminobenzonitrile is used as asubstrate, the fourth nimesulide derivative with higher bioavailability is generated through a series of redox reactions, four novel nimesulide derivatives are successfully prepared, all the four compounds are soluble in water, the solubility is better than that of the nimesulide, and better absorption rate and bioavailability are provided, and the hepatotoxicity of the four derivatives is not obviously different from that of the nimesulide at normal dose, and only one derivative is enhanced in liver toxicity at overdose. The inhibitory effect on BEL-7402 is no less than or even stronger thanthat of nimesulide when the four derivatives are co-cultured with CIK cells. The four nimesulide derivatives can be used for preparing drugs for inhibiting human liver cancer cells.
This letter presents synthesis and structure-activity relationship study of sulfonamide derivatives as inhibitors of Human Uric Acid Transporter 1 (hURAT1). Among all tested sulfonamide derivatives, compounds 9b, 16i and 19b exhibited excellent inhibition activity with IC50 value of 10, 2, and 83?nM, respectively. In addition, compounds 9b and 19b demonstrated moderate PK profile in rats.
The process development for the synthesis of BMS-986020 (1) via a palladium catalyzed tandem borylation/Suzuki reaction is described. Evaluation of conditions culminated in an efficient borylation procedure using tetrahydroxydiboron followed by a tandem Suzuki reaction employing the same commercially available palladium catalyst for both steps. This methodology addressed shortcomings of early synthetic routes and was ultimately used for the multikilogram scale synthesis of the active pharmaceutical ingredient 1. Further evaluation of the borylation reaction showed useful reactivity with a range of substituted aryl bromides and iodides as coupling partners. These findings represent a practical, efficient, mild, and scalable method for borylation.
C17H16BNO2

4-tert-Butylcatechol

4-cyanophenylboronic acid
| Conditions | Yield |
|---|---|
|
In
chloroform-d1;
at 25 ℃;
Equilibrium constant;
Sealed tube;
|
4-bromobenzenecarbonitrile


4-cyanophenylboronic acid
| Conditions | Yield |
|---|---|
|
4-bromobenzenecarbonitrile;
With
TurboGrignard;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran;
at 0 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran;
at 0 ℃;
Inert atmosphere;
|
72% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
diethyl ether; hexane;
at -78 ℃;
for 1h;
With
Triisopropyl borate;
In
diethyl ether; hexane;
at -78 ℃;
for 1h;
|
65% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -70 ℃;
for 1.5h;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -70 - 20 ℃;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexane;
|
63% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium; 3 A molecular sieve;
In
tetrahydrofuran; hexanes;
at -105 - -93 ℃;
for 0.25h;
With
Trimethyl borate;
In
tetrahydrofuran; hexanes;
at -105 - 20 ℃;
for 2.43333h;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexanes;
pH=2.2;
|
59.9% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran; hexanes;
at -105 - -93 ℃;
for 0.25h;
With
Trimethyl borate;
In
tetrahydrofuran; hexanes;
at -105 - 20 ℃;
for 2.43333h;
With
hydrogenchloride;
In
tetrahydrofuran; hexanes; dichloromethane; water;
pH=2.2;
|
59.9% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -105 - -93 ℃;
for 0.25h;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -100 - -72 ℃;
for 0.133333h;
With
hydrogenchloride;
more than 3 stages;
|
59.9% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
With
Trimethyl borate;
In
tetrahydrofuran;
at 20 ℃;
for 20h;
Further stages.;
|
37% |
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran;
at -85 ℃;
With
Triisopropyl borate;
In
tetrahydrofuran;
at -85 - 20 ℃;
for 1.5h;
With
hydrogenchloride;
for 2.5h;
Further stages.;
|
|
|
With
n-butyllithium; Triisopropyl borate;
In
tetrahydrofuran; hexane; toluene;
at -70 - -20 ℃;
|
91 % Chromat. |
|
4-bromobenzenecarbonitrile;
With
metallating reagent;
With
Triisopropyl borate;
Further stages.;
|
|
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran;
at -100 ℃;
for 0.166667h;
Inert atmosphere;
With
Triisopropyl borate;
In
tetrahydrofuran;
at -100 - 20 ℃;
for 1h;
Inert atmosphere;
With
water; ammonium chloride;
In
tetrahydrofuran;
for 0.5h;
|
|
|
4-bromobenzenecarbonitrile;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -110 - -100 ℃;
for 1h;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
at -100 - 20 ℃;
Inert atmosphere;
With
hydrogenchloride; water;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 1h;
Inert atmosphere;
|
|
|
With
n-butyllithium; Trimethyl borate;
In
tetrahydrofuran;
at -100 ℃;
|
|
|
Multi-step reaction with 2 steps
1: n-butyllithium
2: hydrogenchloride; water / 0.5 h
With
hydrogenchloride; n-butyllithium; water;
|
|
|
With
tetrahydroxydiboron; 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); N-ethyl-N,N-diisopropylamine; triphenylphosphine;
In
ethanol;
at 20 ℃;
for 4h;
Inert atmosphere;
Sealed tube;
|
|
|
Multi-step reaction with 2 steps
1: n-butyllithium / tetrahydrofuran
2: oxonium
With
n-butyllithium; oxonium;
In
tetrahydrofuran;
|
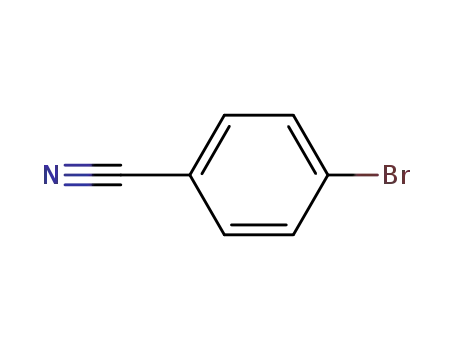
4-bromobenzenecarbonitrile
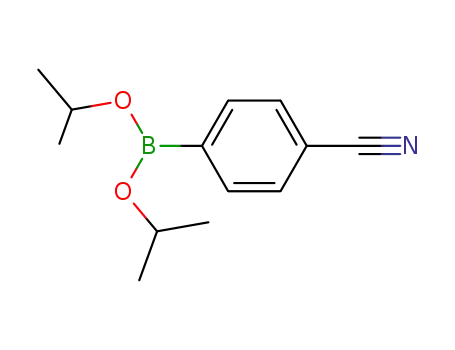
di-isopropyl 4-cyanophenylboronate
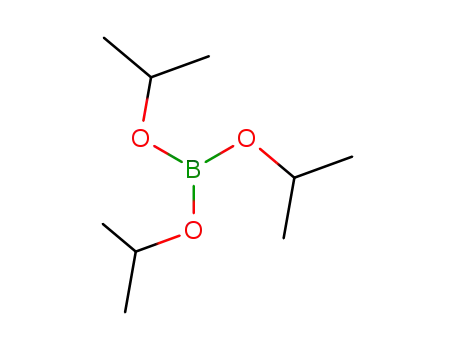
Triisopropyl borate
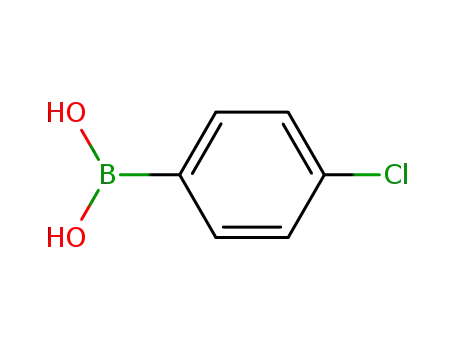
4-Chlorophenylboronic acid
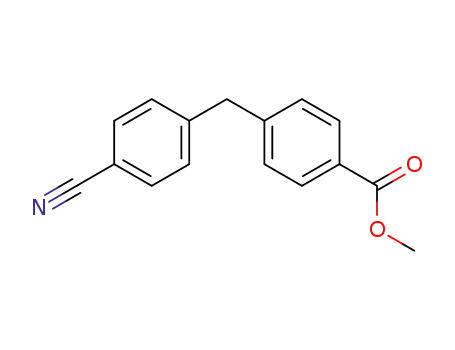
methyl 4-(4-cyanobenzyl)benzoate
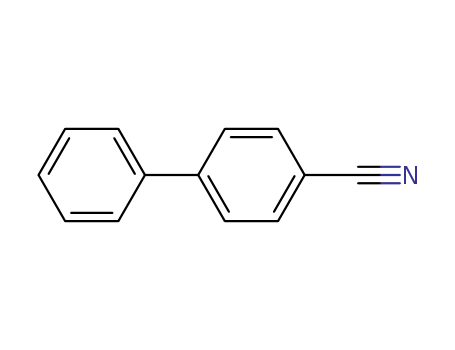
4-cyano-1,1'-biphenyl
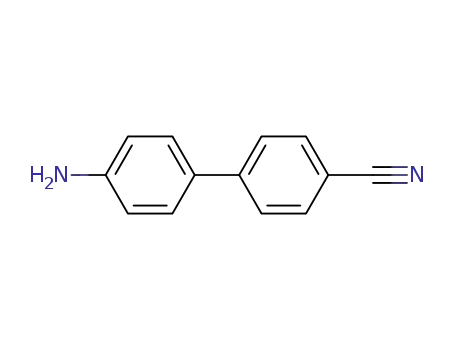
4-amino-4'-cyanobiphenyl
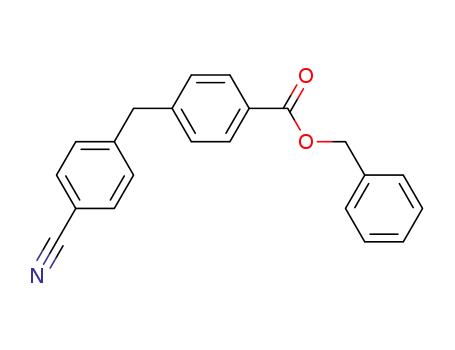
Benzyl 4-[(4-cyanophenyl)methyl]benzoate
CAS:7065-92-1
CAS:762287-57-0
Molecular Formula:C7H8BClO3
Molecular Weight:186.4
CAS:63503-60-6