Your Location:Home >Products >OLED intermediates >Boric acids >63503-60-6
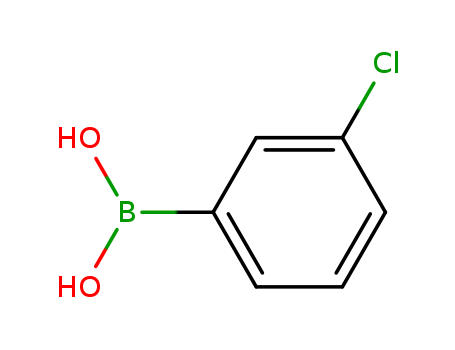

Product Details
Chemical Properties
white to light yellow-green crystalline powder or
Uses
suzuki reaction
Uses
It is employed as a reactant involved in 1,4-conjugate addition reactions with ethenesulfonamides to form arylethanesulfonamides, Suzuki-Miyaura reactions with dibromotrifluoromethylbenzene, cross-coupling reactions with diazoesters3 or potassium cyanate. It is involved in the synthesis of biarylketones and phthalides and synthesis of inhibitors including PDE4 inhibitors6 among others.
Uses
Reactant involved in: 1,4-conjugate addition reactions with ethenesulfonamides to form arylethanesulfonamidesSuzuki-Miyaura reactions with dibromotrifluoromethylbenzeneCross-coupling reactions with diazoesters or potassium cyanateSynthesis of biarylketones and phthalidesSynthesis of inhibitors including PDE4 inhibitors among others
InChI:InChI=1/C6H6BClO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4,9-10H
The invention belongs to the technical field of chemical synthesis, and particularly discloses a preparation method of 3-chlorophenylboronic acid. The preparation method comprises the following steps:preparing 3-chlorophenylboronic acid by using m-bromochlorobenzene as a raw material, preparing a Grignard reaction reagent, reacting with borate to generate 3-chlorophenylborate, and carrying out acid hydrolysis on the 3-chlorophenylborate to obtain a crude product; carrying out gradient cooling crystallization, filtering, ether solvent pulping and filter cake washing on the crude product to obtain a product with the purity of 99.5% or above and the single impurity content smaller than 0.1%, meeting the purity requirement of the bulk drug production process for the medical intermediate. Besides, the total yield of the 3-chlorophenylboronic acid synthesized by the method is over 70 percent and is obviously superior to the yield of the 3-chlorophenylboronic acid synthesized by the conventional Grignard reagent method; the intermediate product Grignard reagent can be directly used for the next reaction without separation, so that the process is effectively simplified, the sewage discharge is reduced, and the cost is reduced.
The invention relates to the technical field of chemical synthesis, and particularly discloses a preparation method of monohalogenated phenylboronic acid. The preparation method comprises the following steps of: by taking dihalogenated benzene as a raw material and a mixture of lithium salt and alkaline ionic liquid as a catalyst, carrying out Grignard exchange with R1MgCl to generate monohalogenated phenyl magnesium chloride, reacting with B (OR) 3 to generate monohalogenated phenyl borate, and hydrolyzing under acidic conditions to obtain monohalogenated phenylboronic acid. The HPLC (High Performance Liquid Chromatography) content of the monohalogenated phenylboronic acid prepared by the method is greater than 99.5%; the total yield of the product is greater than 80%, the contents of monohalogenated phenylboronic acid and phenyldiboronic acid impurities of another halogen are both less than 0.003%, the requirements of modern fine chemical synthesis are completely met, the raw materials are easily available, the operation is simple, the safety is high, and the industrial production of monohalogenated phenylboronic acid is realized.
The invention provides a synthesis method of 3-chlorobenzeneboronic acid. The synthesis method comprises the following steps: with m-dichlorobenzene as a starting material and iodine particles or 1,2-dibromoethane as an initiator, reacting with magnesium in the presence of an organic solvent to obtain a 3-chlorophenylmagnesium chloride reaction solution; then, reacting with trimethyl borate in anorganic solvent to obtain dimethyl 3-chlorobenzeneborate; then, hydrolyzing by using an acid aqueous solution, extracting by using an organic solvent and crystallizing by using an organic solvent to obtain the 3-chlorophenylboronic acid with the purity being higher than or equal to 98.5%. By the synthesis method, raw materials are cheap and easy to obtain, reaction steps are few, the production cost is low, a technology is simple, the pollution is small, and achievement of the industrial production is facilitated.
In this structure-activity relationship study, the influence of aryl substituents at position 5 or 6 on the pharmacological profile of the partial PPARγ agonist 4‘-((2-propyl-1H-benzo[d]imidazol-1-yl)methyl)-[1,1‘-biphenyl]-2-carboxylic acid was investigated. This lead was previously identified as the essential part of telmisartan to induce PPARγ activation. Para-OCH3-phenyl substitution strongly increased potency and efficacy independent of the position. Both compounds represent full agonists because of strong hydrophobic contacts with the amino acid Phe363 in the ligand binding domain. Partial agonists with higher potency than telmisartan or the lead were obtained with OH or Cl substituents at the phenyl ring. Molecular modeling suggested additional hydrogen or halogen bonds with Phe360 located at helix 7. It is assumed that these interactions fix helix 7, thereby promoting a partial agonist conformation of the receptor. The theoretical considerations correlate very well with the results from the luciferase transactivation assay using hPPARγ-LBD as well as those from a time-resolved fluorescent resonance energy transfer (TR-FRET) assay in which the coactivator (TRAP220, PGC-1α) recruitment and corepressor (NCoR1) release pattern was investigated.
C8H10BClO2

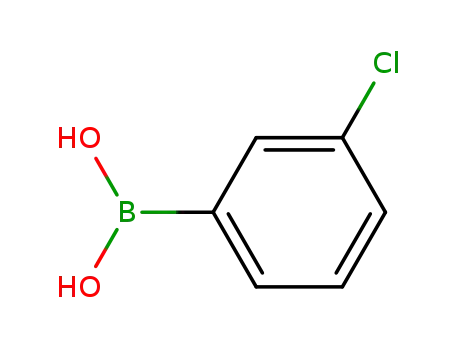
3-chlorophenylboronic acid
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
ethanol; toluene;
at 0 - 40 ℃;
Solvent;
Reagent/catalyst;
|
57% |
|
With
water;
at 98 ℃;
for 2h;
|
14.37 g |
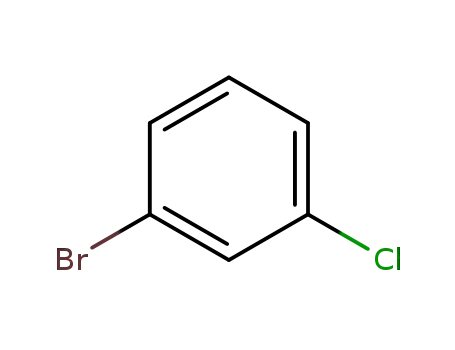
1-bromo-3-chlorobenzene


3-chlorophenylboronic acid
| Conditions | Yield |
|---|---|
|
1-bromo-3-chlorobenzene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
Inert atmosphere;
With
Trimethyl borate;
In
tetrahydrofuran; hexane;
Inert atmosphere;
|
57% |
|
With
Trimethyl borate;
|
|
|
With
Trimethyl borate;
|
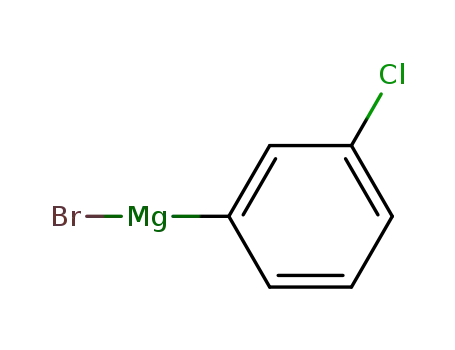
(3-chlorophenyl)magnesium bromide
3-iodochlorobenzene

1-bromo-3-chlorobenzene
borane
tris(3-chlorophenyl)boroxine
2-(3-chloro-phenyl)-[1,3,6,2]dioxazaborocane
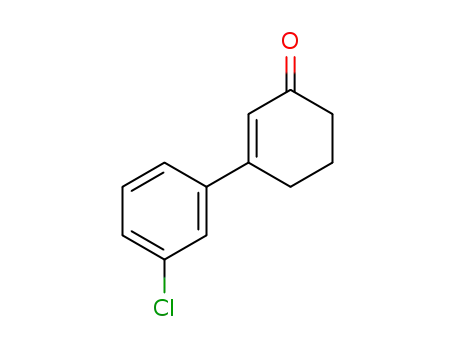
3-(3-chlorophenyl)cyclohex-2-enone
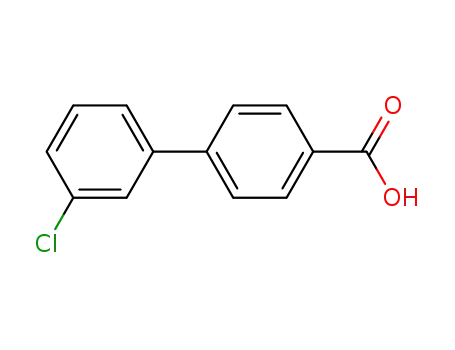
3'-chloro-[1,1'-biphenyl]-4-carboxylic acid
CAS:400607-16-1
CAS:100953-52-4
CAS:126747-14-6