Your Location:Home >Products >Functional intermediates >22082-99-1


Product Details
Chemical Properties
White powder
Uses
2-(4-Bromophenyl)naphthalene is used in the preparation of α-Phosphonosulfonate compounds which inhibit the enzyme squalene and thereby inhibit cholesterol biosynthesis.
InChI:InChI=1/C16H11Br/c17-16-9-7-13(8-10-16)15-6-5-12-3-1-2-4-14(12)11-15/h1-11H
The MeOTf/KI-catalyzed synthesis of 2-arylnaphthalene derivatives from aryl ethylene oxides in alcohol under ambient conditions is described. The present protocol has a higher atom efficiency and wider substrate applicability with excellent yields. The reaction proceeded using the aryl ethylene oxides to give 2-arylnaphthalenes either in homo-coupling or in cross-coupling. The reaction could also be carried out at the gram scale in minutes.
A double-deck silsesquioxane derivative represented by chemical formula 1, a method for preparing the same, and an organic light emitting diode including the derivative are provided. Chemical Formula 1. R is the same as in the above formula. 1 Is a substituted or unsubstituted alkyl group, or a substituted or unsubstituted aryl group. R2 is possible. . Or. Me. (by machine translation)
The invention belongs to the technical field of organic synthesis and catalysis, and particularly relates to a preparation method for synthesizing 9-(naphthalene-1-yl)-10-(4-(naphthalene-2-yl)phenyl)anthracene through a five-step reaction, and a purification method. The method provided by the invention has the advantages of less catalyst dosage, high synthesis yield, less reaction by-products (impurities) (the content of removed boric acid products is less than 1%, and boric acid self-coupling products are not generated), high product purity (the HPLC purity is greater than or equal to 99.99%)and the like, and can be directly applied to OLED terminal materials of devices, and is simple, easy to operate and suitable for large-scale industrial production.
Two benzyne-enabled desulfurization reactions have been demonstrated which convert diaryl sulfoxides and heteroaryl sulfoxides to biaryls and desulfurized heteroarenes, respectively. The reaction accessing biaryls tolerates a variety of functional groups, such as halides, pseudohalides, and carbonyls. Mechanistic studies reveal that both reactions proceed via a common assembly process but divergent disassemblies of the generated tetraaryl(heteroaryl) sulfuranes.
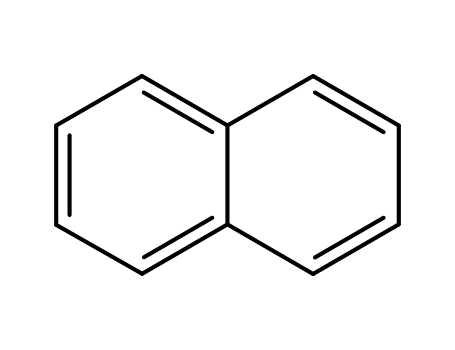
naphthalene

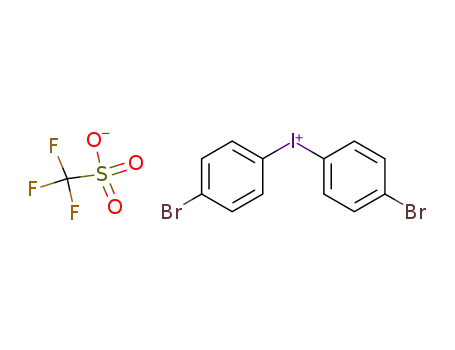
bis(4-bromophenyl)iodonium triflate


2-(4-bromophenyl)naphthalene


1-(4-bromo-phenyl)-naphthalene
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
at 150 ℃;
for 1h;
Overall yield = 69 %; Overall yield = 98 mg;
Inert atmosphere;
Microwave irradiation;
|
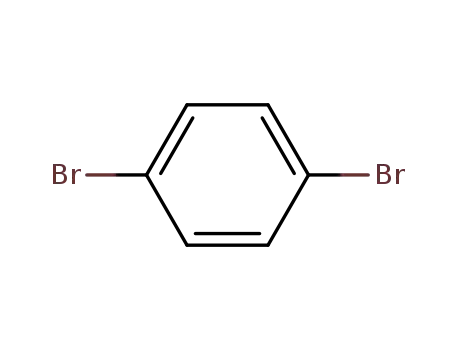
1.4-dibromobenzene

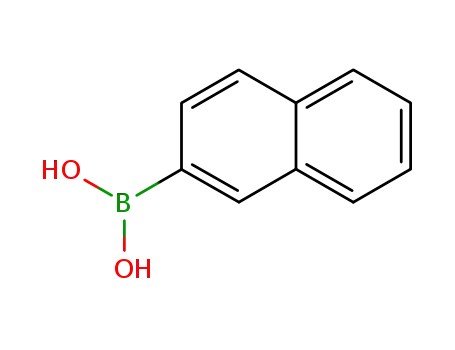
naphthalene-2-boronic acid


2-(4-bromophenyl)naphthalene
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran;
at 65 ℃;
for 18h;
|
70% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran;
for 24h;
Reflux;
Inert atmosphere;
|
65% |
|
With
potassium carbonate;
tetrakis(triphenylphosphine) palladium(0);
In
tetrahydrofuran; water;
for 24h;
Heating / reflux;
|
47% |
|
With
sodium carbonate;
tetrakis(triphenylphosphine) palladium(0);
In
1,2-dimethoxyethane; water;
at 80 ℃;
for 3h;
Inert atmosphere;
|
|
|
With
tetrakis(triphenylphosphine) palladium(0); sodium carbonate;
In
1,2-dimethoxyethane; water;
at 80 ℃;
for 3h;
Inert atmosphere;
|
10 g |
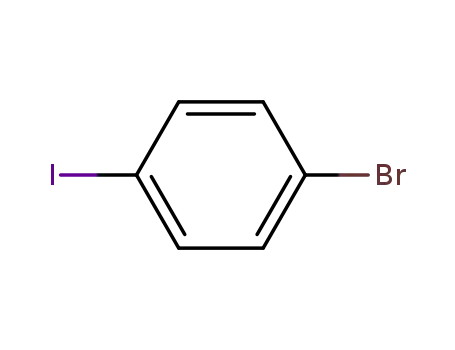
1,4-bromoiodobenzene

naphthalene-2-boronic acid
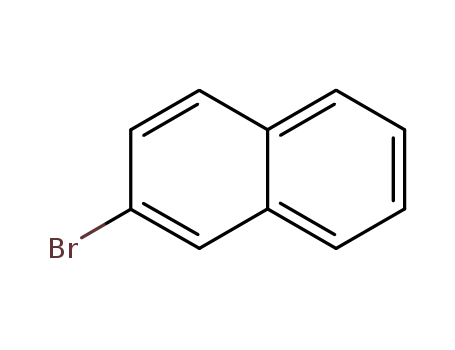
2-bromonaphthalene
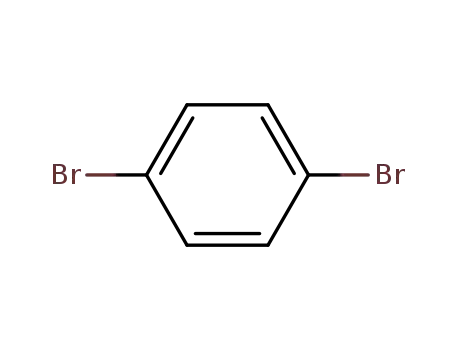
1.4-dibromobenzene
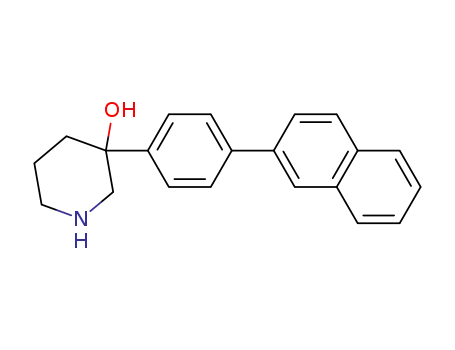
3-Hydroxy-3-(4-naphth-2-yl-phenyl)piperidine
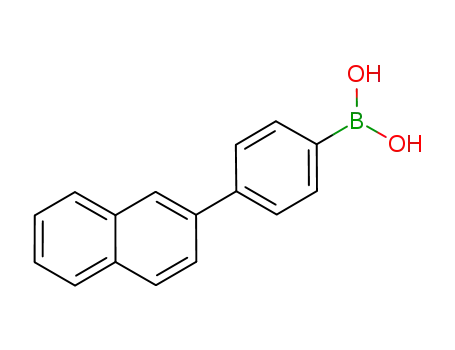
4-(naphthalene-2-yl)phenylboronic acid pinacol ester
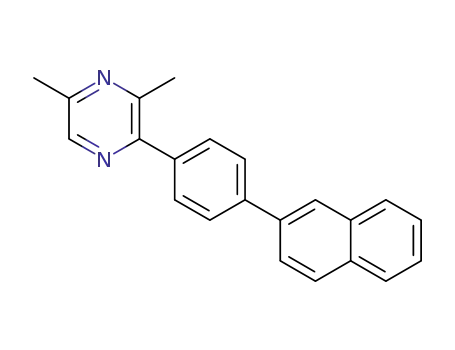
3,5-dimethyl-2-(4-naphthalen-2-yl-phenyl)pyrazine
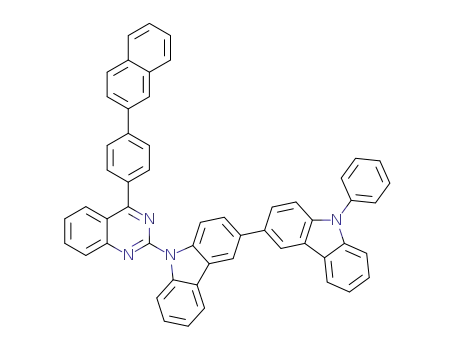
C54H34N4
CAS:1228468-73-2
CAS:1609484-45-8
CAS:1700-02-3
Molecular Formula:C<sub>9</sub>H<sub>5</sub>C<sub>l2</sub>N<sub>3</sub>
Molecular Weight:226.06
CAS:3741-77-3