Your Location:Home >Products >Functional intermediates >1700-02-3

Product Details
|
1700-02-3 Name |
|
|
Name |
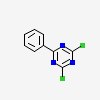
2,4-Dichloro-6-phenyl-1,3,5-triazine |
|
Synonym |
2,4-Dichloro-6-phenyl-1,3,5-triazine 98%;4,6-DICHLORO-2-PHENYL TRIAZINE;1,3,5-TRIAZINE-2,4-DICHLORO, 6-PHENYL-;2,4-DICHLORO-6-PHENYL-1,3,5-TRIAZINE;2-[(4-AMINO-3-CHLORO)BENZOYL]BENZOIC ACID;2,4-Dichloro-Phenyl-1,3,5-Triazine;2-Phenyl-4,6-dichlorotriazine;4,6-Dichloro-2-phenyl-1,3,5-triazine |
|
1700-02-3 Chemical & Physical Properties |
|
|
Melting point |
121 °C |
|
Boiling point |
427.5±28.0 °C at 760 mmHg |
|
Density |
1.4±0.1 g/cm3 |
|
Molecular Formula |
C9H5Cl2N3 |
|
Molecular Weight |
226.062 |
|
Flash Point |
244.9±9.6 °C |
|
PSA |
38.67000 |
|
LogP |
3.18 |
|
Exact Mass |
224.986053 |
|
Vapour Pressure |
0.0±1.0 mmHg at 25°C |
|
Index of Refraction |
1.610 |
2,4-Dichloro-6-phenyl-1,3,5-triazine,White to Orange to Green powder to crystal, is a heterocyclic derivative and can be used as intermediate of synthetic materials.
InChI:InChI=1/C9H5Cl2N3/c10-8-12-7(13-9(11)14-8)6-4-2-1-3-5-6/h1-5H
A series of molecules; tBuCz1SiTrz, tBuCz2SiTrz and tBuCz3SiTrz, which contain carbazole unit as hole-transporting group (donor-D) and triazine unit as electron transporting group (acceptor-A) were synthesized and characterized as high-triplet energy (>2.9 eV), solution-processable bipolar emitting materials. Photophysical, electrochemical and computational characterizations addressed that tBuCz2SiTrz has the weakest ICT character, highest photoluminescence quantum yield (PLQY) and charge balance.
Four new europium complexes were prepared by treating europium(III) trifluoro(thenoyl)acetonate trihydrate with new tridentate ligands, based on dipyrazolyltriazine and utilized as emitting materials in electroluminescent devices.
The invention belongs to the technical field of organic electroluminescent devices, and discloses a novel electron-deficient acceptor material and an application thereof in organic electroluminescent devices.
UV stabilizers, such as Tinuvin 1577, are organic additives that are used in the polymer industry to suppress polymer photodegradation, however leaching of the stabilizers from polymers is a significant practical issue which limits the effectiveness of the stabilizers and restricts polymer lifetimes.
In this study, we determined the crystal structure of an engineered human adenosine A2A receptor bound to a partial agonist and compared it to structures cocrystallized with either a full agonist or an antagonist/inverse agonist.
The invention relates to an organic compound. The structure of the organic compound comprises a formula I. When the organic compound provided by the invention is used for a light-emitting layer of anorganic electroluminescent device, the device efficiency of the device can be effectively improved, and the service life of the organic electroluminescent device is prolonged.
1,3,5-trichloro-2,4,6-triazine

phenylmagnesium bromide

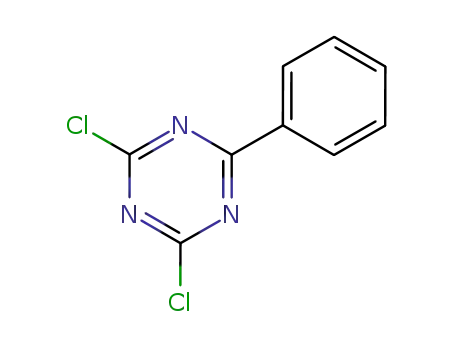
2,4-dichloro-6-phenyl-1,3,5-triazine

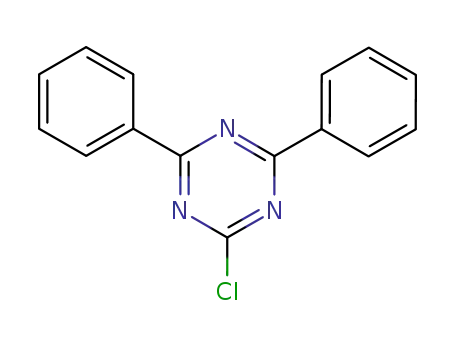
2-chloro-4,6-diphenyl-1,3,5-triazine
| Conditions | Yield |
|---|---|
|
With diethyl ether;
|

1,3,5-trichloro-2,4,6-triazine

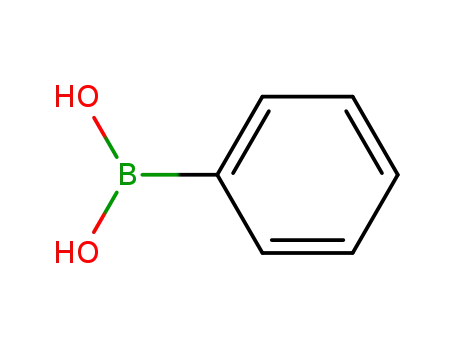
phenylboronic acid


2,4-dichloro-6-phenyl-1,3,5-triazine
| Conditions | Yield |
|---|---|
|
With potassium carbonate; In ethanol; water; at 27 - 30 ℃; for 1h; Irradiation;
|
92% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 24h;
|
85% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; toluene; for 9h; Reflux;
|
84% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; toluene; for 9h; Reflux;
|
84% |
|
With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate; In toluene; at 60 ℃; for 12h; Inert atmosphere; Schlenk technique;
|
82% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 10h; Inert atmosphere;
|
80.4% |
|
With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate; In toluene; for 10h; Inert atmosphere; Reflux;
|
80% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 10h;
|
75.4% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 10h; Inert atmosphere;
|
75.4% |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In tetrahydrofuran; water; at 80 ℃; for 10h; Inert atmosphere;
|
75.4% |
|
With tetrakis(triphenylphosphine) palladium(0); In tetrahydrofuran; water; at 90 ℃;
|
60% |
|
With tetrakis(triphenylphosphine) palladium(0); sodium hydroxide; In tetrahydrofuran; water;
|

1,3,5-trichloro-2,4,6-triazine
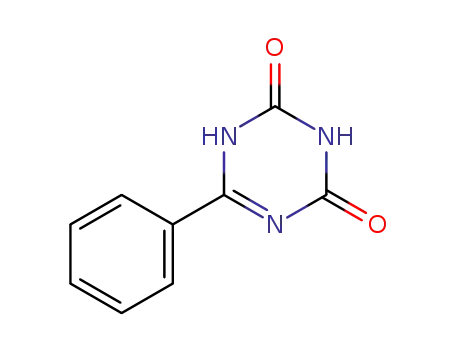
6-phenyl-1,3,5-triazine-2,4(1H,3H)-dione
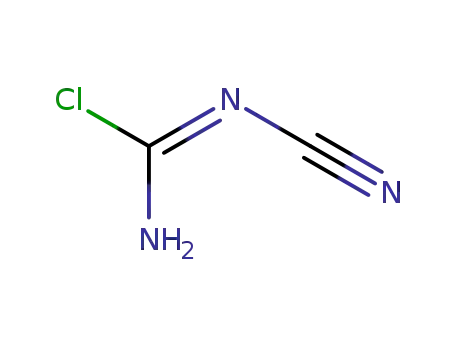
N-Cyanochloroformamidine
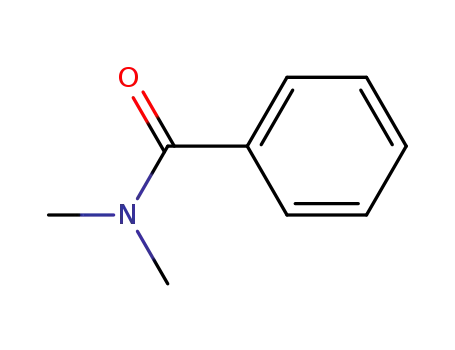
N,N-dimethylbenzamide
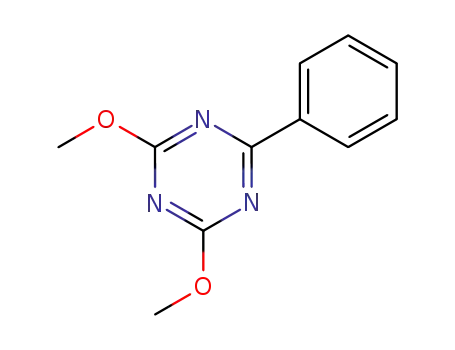
2,4-dimethoxy-6-phenyl-1,3,5-triazine
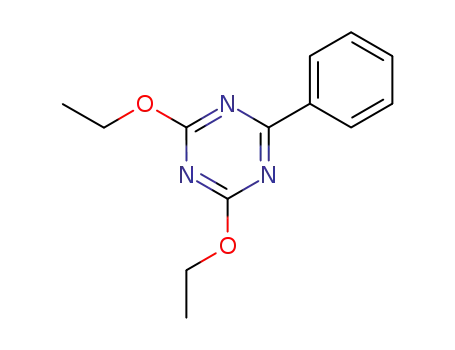
2,4-diethoxy-6-phenyl-[1,3,5]triazine
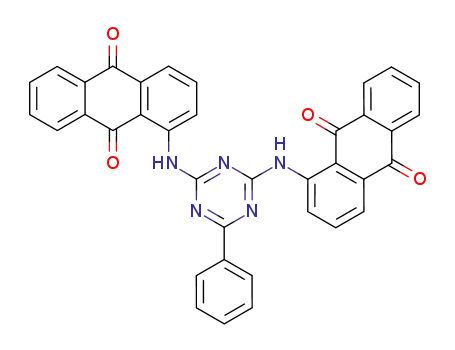
1,1'-(6-phenyl-[1,3,5]triazine-2,4-diyldiamino)-bis-anthraquinone
2,6-difluoro-4-phenyl-s-triazine
CAS:14647-23-5
Molecular Formula:C26H24Cl2NiP2
Molecular Weight:528
CAS:2489-86-3
CAS:22082-99-1