Your Location:Home >Products >OLED intermediates >Boric acids >864377-33-3

Product Details
|
Chemical Properties |
off-white solid |
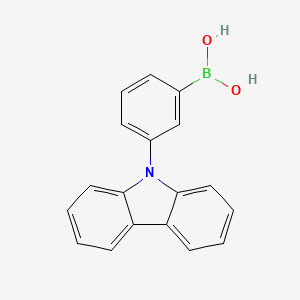
| Description | 3-(9H-Carbazol-9-yl)phenylboronic acid is an organic compound consists of a carbazole group attached to a phenyl group, which is further functionalized with a boronic acid group. This compound has potential applications in organic electronics and materials science due to the unique properties imparted by the carbazole moiety and the boronic acid group. It can be used as a building block for the synthesis of various functional materials and as a component in the fabrication of organic electronic devices, such as organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs). |
|
Uses |
suzuki reaction |
Isomeric SMILES: B(C1=CC(=CC=C1)N2C3=CC=CC=C3C4=CC=CC=C42)(O)O
InChIKey: IDQUIFLAFFZYEX-UHFFFAOYSA-N
InChI: InChI=1S/C18H14BNO2/c21-19(22)13-6-5-7-14(12-13)20-17-10-3-1-8-15(17)16-9-2-4-11-18(16)20/h1-12,21-22H
The present invention relates to an orga...
The present invention relates to an orga...
The invention provides an organic electr...
The invention discloses an organic elect...
9-(3-bromophenyl)carbazole

Trimethyl borate

3-(9H-carbazol-9-yl)phenylboronic acid
| Conditions | Yield |
|---|---|
|
9-(3-bromophenyl)carbazole; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 1h;
Trimethyl borate; In tetrahydrofuran; at 20 ℃;
|
83% |
|
9-(3-bromophenyl)carbazole; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 1h;
Trimethyl borate; In tetrahydrofuran; at 20 ℃;
|
72% |
|
9-(3-bromophenyl)carbazole; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
Trimethyl borate; In tetrahydrofuran; hexane; for 2h;
|
67% |
9-(3-bromophenyl)carbazole

3-(9H-carbazol-9-yl)phenylboronic acid
| Conditions | Yield |
|---|---|
|
9-(3-bromophenyl)carbazole; With n-butyllithium; In tetrahydrofuran; hexane; at -65 - -60 ℃; for 0.783333h; Cooling in ethanol bath;
With Triisopropyl borate; In tetrahydrofuran; hexane; at 20 ℃; for 2.2h;
With hydrogenchloride; water; In tetrahydrofuran; hexane; for 0.5h;
|
|
|
Multi-step reaction with 2 steps
1.1: n-butyllithium / tetrahydrofuran; n-heptane / 1 h / -78 °C / Inert atmosphere
1.2: 4 h / -78 - 0 °C
2.1: hydrogenchloride; water / tetrahydrofuran; n-heptane; dichloromethane
With hydrogenchloride; n-butyllithium; water; In tetrahydrofuran; n-heptane; dichloromethane;
|
9-(3-bromophenyl)carbazole
9H-carbazole
Trimethyl borate
1,3-dibromobenzene
3'-(9H-carbazol-9-yl)-[1,1'-biphenyl]-4-amine
C30H21BrN2
2,6-bis(3-(9H-carbazol-9-yl)phenyl)-N,N-diphenylpyridin-4-amine
9,9'-(3',3-(4-phenyl-4H-1,2,4-triazole-3,5-diyl)bis(biphenyl-3',3-diyl))bis(9H-carbazole)
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:6299-16-7
CAS:1257220-52-2