Your Location:Home >Products >OLED intermediates >Carbazoles >22034-43-1
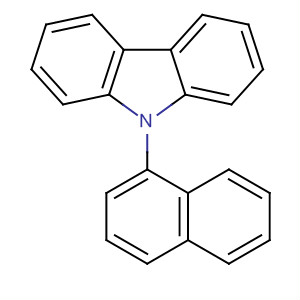

Product Details
Uses
9-(Naphthalen-1-yl)-9H-carbazole is a useful research chemical.
InChI:InChI=1/C22H15N/c1-2-10-17-16(8-1)9-7-15-20(17)23-21-13-5-3-11-18(21)19-12-4-6-14-22(19)23/h1-15H
A novel π-conjugated small molecule VNCO, 2,5-bis{3′-[3″-vinyl-9″-(α-naphthyl)carbazolyl]phenyl} -1,3,4-oxadiazole, containing hole-transporting carbazole moieties, electron-injecting 1,3,4-oxadiazole moieties and chromophore naphthalene was designed and synthesized by Wittig reaction of 2,5-bis(3-tolylene-triphenylphosphonium bromide)-1,3,4-oxadiazole and 3-formyl-9-(α-naphthyl)carbazole. The UV-vis absorption, fluorescence excitation and emission spectra have been obtained in solution for VNCO. The photoluminescence (PL) of VNCO were examined in different solvents and the luminescence quantum yield was 0.746 in chloroform. It emitted blue and blue-green light, with the band gap of 3.30 eV estimated from the onset absorption. In addition, the light-emitting can be quenched by both electron donor (N,N-dimethylaniline) and electron acceptor (dimethylterephalate). Furthermore, the molecular interactions of VNCO with fullerene (C60) or carbon nanotubes (CNTs) were also carefully investigated.
A new ambipolar fluorophore, 3,6-di(4,4′-dimethoxydiphenylaminyl)-9-(1-naphthyl)carbazole, was synthesized and its physical properties were studied by differential scanning calorimetry, thermogravimetric analysis, UV-vis absorption, luminescence and photo
The present invention provides photocatalysts, methods for their preparation, and methods for preparing linear polymers with high propagation rate.
PROBLEM TO BE SOLVED: To provide a method for producing 9-(1-naphthyl)-9H-carbazole derivatives. SOLUTION: The production method includes causing a reaction to occur between a 9H-carbazole derivative and 1-halonaphthalene in the presence of a palladium compound, a biarylphosphine represented by formula (4), and a lithium base. (R3: a C4-10 linear, branched or cyclic alkyl. R4 and R5: H or a benzene ring formed together with bonded C. R6, R7, R8, R9 and R10: H, a C1-3 alkyl or the like). SELECTED DRAWING: None COPYRIGHT: (C)2020,JPOandINPIT
Palladium-catalysed Buchwald–Hartwig amination of ortho-substituted hindered aryl bromides or chlorides with 9H-carbazole has been investigated. In the amination of 1-bromo- or chloronaphthalene with 9H-carbazole, the combined use of Pd2(dba)s
PROBLEM TO BE SOLVED: To efficiently produce 9-(2-substituted phenyl)-9H-carbazole. SOLUTION: A 9-(2-substituted phenyl)-9H-carbazole is produced by causing a reaction to occur between a 9H-carbazole derivative and 2-substituted-1-halobenzene in the prese
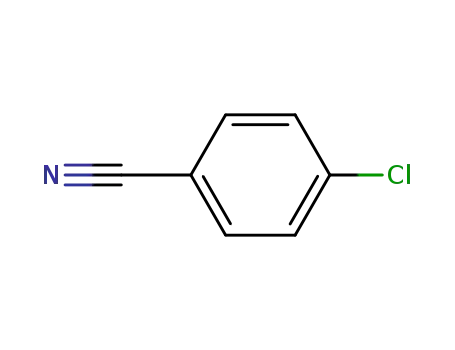
4-Cyanochlorobenzene

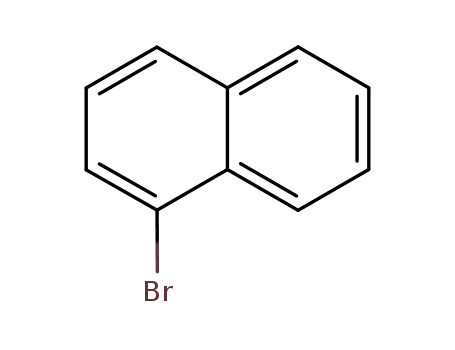
1-Bromonaphthalene

Cu2[P(m-tol)3]4(carbazolide)2

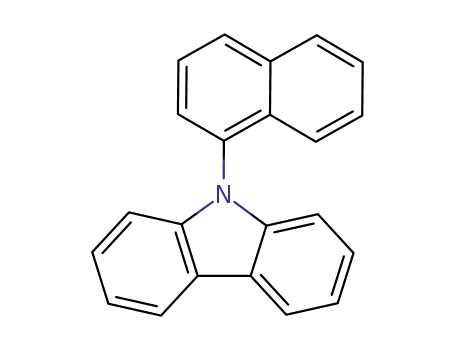
9-(naphthalen-1-yl)-9H-carbazole

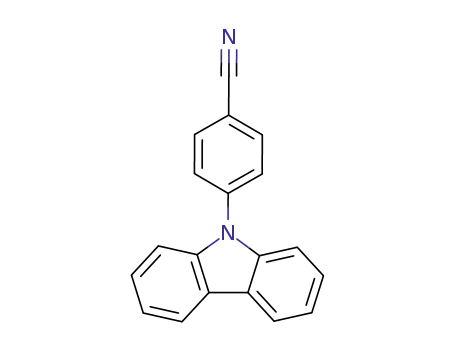
N-(4-cyanophenyl)carbazole
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
at 20 ℃;
Irradiation;
|
24% 44% |

1-Bromonaphthalene

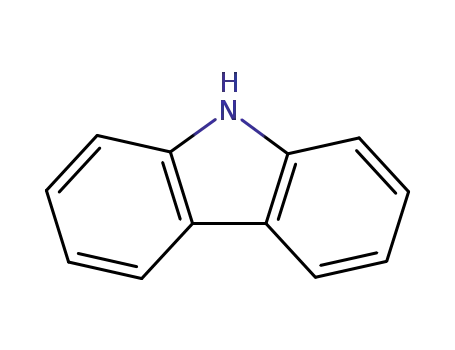
9H-carbazole


9-(naphthalen-1-yl)-9H-carbazole
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); di‐tert‐butyl(2',6'‐dimethoxy‐[1,1'‐biphenyl]‐2‐yl)phosphane; lithium hexamethyldisilazane;
In
5,5-dimethyl-1,3-cyclohexadiene; toluene;
at 140 ℃;
for 15h;
Reagent/catalyst;
Temperature;
Solvent;
Inert atmosphere;
|
94% |
|
With
potassium carbonate;
In
neat (no solvent);
at 200 - 220 ℃;
for 48h;
Inert atmosphere;
|
94.5% |
|
With
copper(l) iodide; 18-crown-6 ether; potassium carbonate;
In
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU);
at 170 - 180 ℃;
for 13.5h;
|
75% |
|
With
18-crown-6 ether; potassium carbonate;
copper(l) iodide;
In
1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone;
at 170 - 180 ℃;
for 19.5h;
|
75% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); N,N′-bis(2,6-bis(diphenylmethyl)-4-methoxyphenyl)imidazol-2-ylidene; lithium hexamethyldisilazane;
In
5,5-dimethyl-1,3-cyclohexadiene; toluene;
at 140 ℃;
for 15h;
Reagent/catalyst;
Solvent;
Catalytic behavior;
Inert atmosphere;
Sealed tube;
|
73% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); N,N′-bis(2,6-bis(diphenylmethyl)-4-methoxyphenyl)imidazol-2-ylidene; lithium hexamethyldisilazane;
In
5,5-dimethyl-1,3-cyclohexadiene; toluene;
at 140 ℃;
for 15h;
Inert atmosphere;
|
73% |
|
With
copper; potassium carbonate;
In
nitrobenzene;
for 10h;
Heating;
|
71% |
|
With
potassium carbonate;
In
nitrobenzene;
for 48h;
Reflux;
|

1-Bromonaphthalene

9H-carbazole
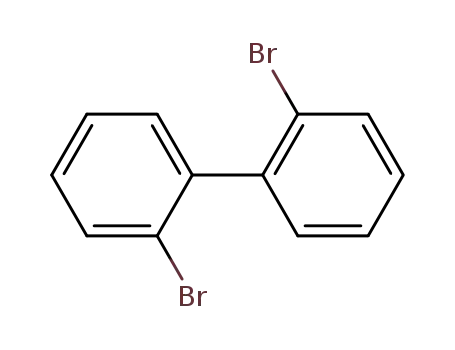
2,2'-dibromobiphenyl
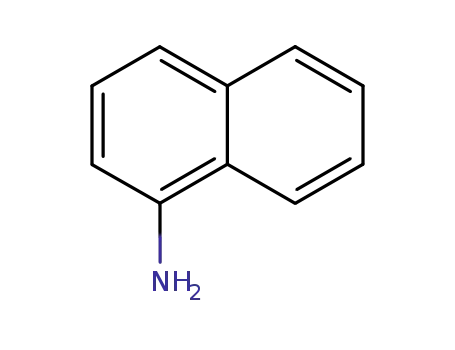
1-amino-naphthalene
3-bromo-9-(naphthalen-1-yl)-9H-carbazole
CAS:1448787-63-0
CAS:538-58-9
CAS:35883-22-8
CAS:419536-33-7
Molecular Formula:C18H14BNO2
Molecular Weight:287.1