Your Location:Home >Products >OLED intermediates >Thiophenes >18794-77-9

Product Details
Chemical Properties
Light yellow liquid
Chemical Properties
Colorless to pale yellow liquid; meat-like aroma
Occurrence
Reported found in beef, cranberry, mushroom and turkey.
Uses
2-n-Hexylthiophene is used as a important raw material and intermediate used in organic synthesis, pharmaceuticals, agrochemicals.
Aroma threshold values
High strength odor, sulfurous type; recommend smelling in a 0.10% solution or less
InChI:InChI=1/C10H16S/c1-2-3-4-5-7-10-8-6-9-11-10/h6,8-9H,2-5,7H2,1H3
Four thiocyanate-free ruthenium sensitizers (DUY24-DUY27) containing 2-thienylpyridine moiety as cyclometalating core were synthesized for dye-sensitized solar cell (DSC) application. To the best of our knowledge, DUY24-DUY27 are four best-efficiency sens
A synthetic strategy to prepare 2,7- or 4,9-functionalized cyclopenta[hi]aceanthrylenes that are capable of Suzuki cross-coupling reactions is demonstrated. This method has been utilized to create a series of thiophene derivatized compounds that were subsequently used to investigate the role of substitution pattern on the photophysical and electronic properties of cyclopenta[hi]aceanthrylenes. The orthogonal functionalization provides access to unique substitution patterns (e.g., cruciform-like architectures) and materials with small optical band gaps (1.22-1.97 eV).
A new star-shaped donor-acceptor molecule has been synthesized for application as the donor material in solution-processed bulk-heterojunction organic solar cells (OSCs). The molecule consists of a triphenylamine (TPA) unit as the core and a donor unit with three arms containing benzo[1,2,5]thiadiazole (BT) acceptor units and 5,5″-dihexyl-2,2′:3′,2″- terthiophene (tTh) end groups. The molecule, denoted S(TPA-BT-tTh), exhibits a broad absorption band in the wavelength range 300-650 nm and high hole mobility of 1.1×10-4 cm2 V-1 s-1. An OSC device based on S(TPA-BT-tTh) as donor and [6,6]-phenyl C 71-butyric acid methyl ester (PC70BM) as the acceptor (1:3, w/w) exhibited a power conversion efficiency of 2.28% with a short circuit current density of 6.39 mA/cm2 under illumination of AM.1.5, 100 mW/cm2.
Three thiocyanate-free cycloruthenated complexes, DUY24-O, DUY24, and DUY24-Se containing furan, thiophene, and selenophene, respectively, as a part of the cycloruthenated ring, were designed to reveal the function of the chalcogen atom on the physicochemical and photovoltaic performance of the cycloruthenated sensitizers applied in dye-sensitized solar cells (DSCs). The three sensitizers have a similar molecular size; therefore, the effect of molecular dimensions on their photovoltaic performance can be negligible. NMR data, electron-donating resonance effects, optical properties, and the energy levels of the frontier orbitals reveal that the physical/photovoltaic properties of the three sensitizers were affected significantly by the chalcogen atom on the cyclometalated chalcogenophene ring. The λmax (both in ethanol and adsorbed on TiO2), frontier orbital level, and dye loading of thiophene- and selenophene-containing dyes are very close. Nevertheless, DUY24-Se has a higher molar absorption coefficient compared to DUY24; therefore, the DSC based on DUY24-Se has higher efficiency (8.4% under AM1.5 G one-sun and 26% under T5-light at ca. 6000 lux) than that sensitized with the DUY24 dye. These efficiencies are also higher than those (7.9 and 21.6%, respectively) of the cell dyed with N719, fabricated using the same conditions. The better performance of the device sensitized with DUY24-Se compared to DUY24-based cells suggests that selenophene is as good as (or even better than) thiophene to be a part of the cyclometalated ring for thiocyanate-free cycloruthenated sensitizers applied in DSCs. Furan-containing DUY24-O has much worse photovoltaic performance compared to the other two dyes. This is not only because DUY24-O has the shortest λmax, the lowest molar absorption coefficient, and the highest HOMO level but also the lowest dye loading (because of the strong interaction between the oxygen in furan and TiO2, the array of DUY24-O occupies more surface when adsorbed on TiO2) and the fastest charge recombination. The physicochemical and photovoltaic properties as well as the adsorption behavior of the dye on the TiO2 anode for the cycloruthenated sensitizers affected significantly by the chalcogen atom of the chalcogenophene on the cyclometalated ring provide a new strategy to design high-efficiency NCS-free cyclometalated sensitizers for DSCs.
(Matrix Presented) Thiophene dendrons and dendrimers were designed and synthesized using a convergent approach. Metal-mediated coupling reactions were used in the synthesis. A rational approach allowed the formation of αα, ββ, and αβ linkages between the dendrons and thiophene units.
The efficient synthesis of novel β-diketonates equipped with functional carbazolyl moieties and their subsequent transformations in 5-hexyl-thienyl substituted carbazole derivatives is presented by utilizing an effective Stille cross-coupling reaction. Th
Supramolecular nano-composites 15T5N/ZnOx-PNH containing enhanced compatibilities of dendritic dye 15T5N and surface-modified nano-rod ZnOx-PNH were developed in this study. The physical properties of supramolecular nano-composites have been well investigated by theoretical calculation and various measurements, including UV–visible, PL, TEM, TCSPC, FT-IR, and CV. H-bonded nano-composite 15T5N/ZnO30-PNH possessed the saturated quenched fluorscence as dendritic dye 15T5N complexed with surface-modified nano-rod ZnO30-PNH. In contrast to nano-rod mixture 15T5N/ZnO (without surface-modification), more widened visible absorption band to promote more efficient photo-induced charge separation phenomenon was observed in supramolecular nano-composite 15T5N/ZnO30-PNH. Compared with surfactant T-PNH, less efficient electron transfer of surfactant T-PC6 was observed in TCSPC and PL measurements because of the steric hindrance of long alkyl chains (-C6H13) attached to surfactant T-PC6. Therefore, the triple H-bonds between dendritic dye 15T5N and surface-modified nano-rod ZnO30-PNH are verified to result in the desirable effective electron transfer in supramolecular nano-composite 15T5N/ZnO30-PNH.
In the newly designed photoswitchable electron transfer compound 2, our previously published donor-bridge-acceptor system (anthracene-CH2- bithiophene-pyridinium) is modified by incorporation of the photoisomerizable dithienylethene (DTE) as a switching unit. In the open-ring form 2a, excitation of the anthracene donor leads to an intramolecular charge separation proved by identification of the anthracene radical cation in transient absorption spectra (ON state). After photocyclization to the closed-ring isomer (2a → 2b), the intramolecular charge separation is completely suppressed (OFF state). The reversibility of the ON/OFF switching is verified. From UV-vis absorption spectra and cyclovoltammetric studies it is deduced that in the open-ring isomer 2a (ON state) conjugation is markedly restricted within the dithienylethene bridge, whereas in the closed-ring isomer 2b (OFF state) the conjugation is extended over the whole dithienylethenepyridinium (DTEP) subunit. Consequently, in 2 an enlarged conjugation within the bridge is not decisive for photoinduced charge separation. Instead the observed transfer properties can be understood by thermodynamic aspects.
A novel large thiophene-fused polycyclic aromatics 1 based on pyrene 5 has been synthesized, and its structure was confirmed by 1H NMR, 13C NMR, MS, UV-vis and elemental analysis. The key steps of the synthesis involved the Stille cross-coupling reaction and followed by selective β-β oxidative cyclization of pendant thienyl rings by FeCl3 under mild conditions.
The invention discloses an organic small molecule hole transport material based on spiro[fluorene-9,9'-xanthene]-based and a preparation method and application thereof, and the organic small moleculehole transport material takes spiro[fluorene-9,9'-xanthene] as a core, and has good amorphous form and excellent dissolving property; besides, different numbers of thiophene groups are added into sidechains, so that the material is endowed with more excellent physical and photoelectric properties through the characteristics of high electron density, excellent carrier transport capacity, controllable optical and electrochemical properties and the like; in addition, due to modification of a terminal alkyl chain, the dissolvability is improved, the film-forming property of the material is facilitated, and the material is easier to process; meanwhile, the synthesis is simple, raw materials are easy to obtain, and cost is low. The organic small molecule hole transport material is applied to anall-inorganic perovskite solar cell, and the cell efficiency of the organic small molecule hole transport material is higher than the original cell efficiency, which shows that the organic small molecule hole transport material has practical significance for improving the efficiency of the all-inorganic perovskite solar cell.
-
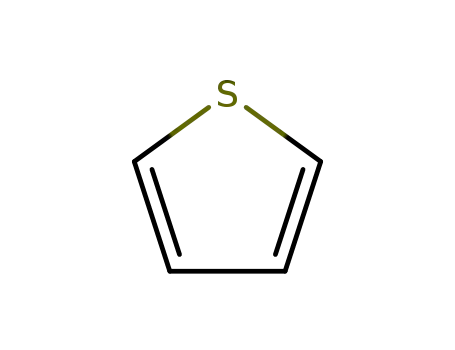
thiophene

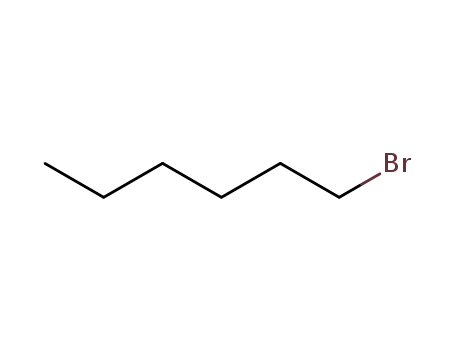
1-bromo-hexane

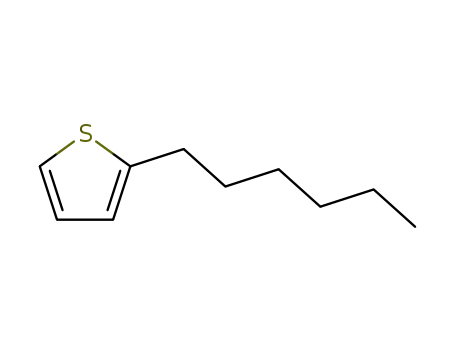
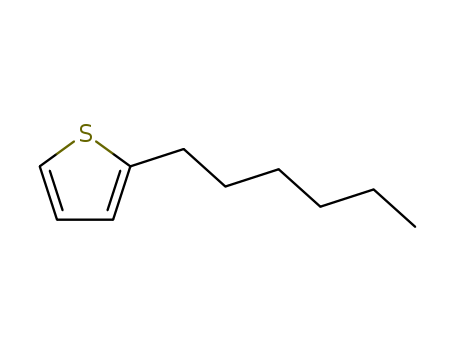
2-hexylthiophene
| Conditions | Yield |
|---|---|
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.75h;
1-bromo-hexane;
In
tetrahydrofuran;
at 20 ℃;
for 3h;
Further stages.;
|
96% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -40 - 20 ℃;
Inert atmosphere;
|
90% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at 20 ℃;
|
89% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
1-bromo-hexane;
In
tetrahydrofuran;
at 20 ℃;
|
86% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
Inert atmosphere;
|
82% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
Inert atmosphere;
|
82% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.833333h;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
Further stages.;
|
81% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 12h;
Inert atmosphere;
|
80% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 12h;
Inert atmosphere;
|
80% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at 0 ℃;
Inert atmosphere;
|
78.5% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 1.16667h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran;
at -78 ℃;
Inert atmosphere;
|
76.1% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 0 ℃;
for 1h;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -40 - 20 ℃;
|
75% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -70 - 0 ℃;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -40 - 20 ℃;
|
75% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
75% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 1.5h;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 15h;
Further stages.;
|
73% |
|
thiophene;
With
n-butyllithium;
Inert atmosphere;
1-bromo-hexane;
Inert atmosphere;
|
70% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 3h;
Inert atmosphere;
|
67% |
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -60 - 0 ℃;
for 1.5h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -60 - 20 ℃;
Inert atmosphere;
|
62% |
|
thiophene;
With
n-butyllithium; N,N,N,N,-tetramethylethylenediamine;
In
tetrahydrofuran; hexane;
at 20 ℃;
for 2h;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
for 8h;
Heating;
|
58% |
|
thiophene;
In
tetrahydrofuran;
at -78 ℃;
for 0.5h;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
for 2h;
1-bromo-hexane;
In
tetrahydrofuran; hexane; water;
at 20 ℃;
for 3h;
|
52% |
|
With
n-butyllithium;
at -78 ℃;
|
|
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
Further stages.;
|
|
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 0.666667h;
Inert atmosphere;
With
potassium tert-butylate;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
1-bromo-hexane;
In
tetrahydrofuran;
at -78 - 20 ℃;
|
|
|
With
n-butyllithium;
In
tetrahydrofuran;
at -60 - 20 ℃;
for 24h;
|
|
|
thiophene;
With
n-butyllithium;
at -78 ℃;
for 0.5h;
1-bromo-hexane;
for 48h;
Reflux;
|
|
|
With
n-butyllithium;
In
tetrahydrofuran;
Inert atmosphere;
Schlenk technique;
|
|
|
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
Inert atmosphere;
|
|
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
1-bromo-hexane;
|
|
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 1h;
Inert atmosphere;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
for 16h;
Inert atmosphere;
|
|
|
thiophene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
for 1h;
1-bromo-hexane;
In
tetrahydrofuran; hexane;
at 20 ℃;
|
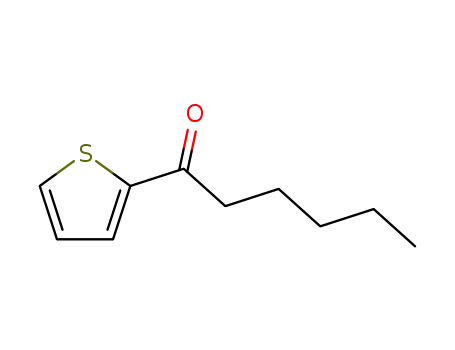
1-(thiophen-2-yl)hexan-1-one


2-hexylthiophene
| Conditions | Yield |
|---|---|
|
With
aluminum (III) chloride; lithium aluminium tetrahydride;
In
diethyl ether;
at 20 ℃;
for 3h;
|
89% |
|
With
cobalt polysulfide; acetic acid;
at 200 - 225 ℃;
under 73550.8 Torr;
Hydrogenation;
|
|
|
With
hydrogenchloride; amalgamated zinc;
|
|
|
ueber das Hydrazon;
|

1-(thiophen-2-yl)hexan-1-one

thiophene
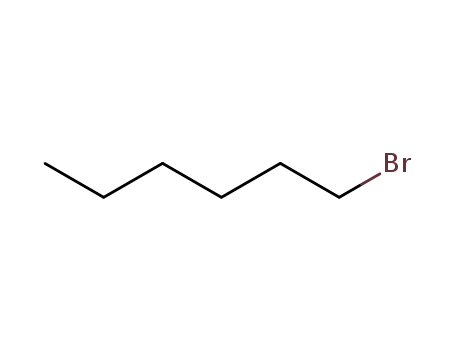
1-bromo-hexane
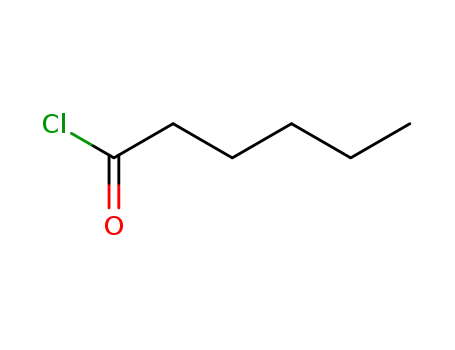
Hexanoyl chloride
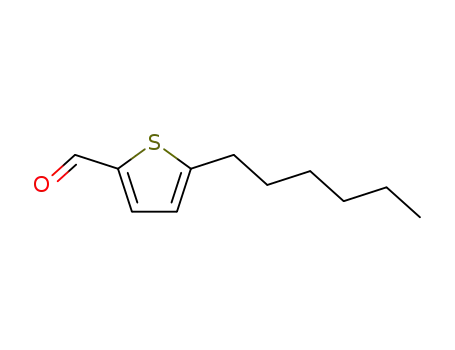
5-hexylthiophene-2-carbaldehyde
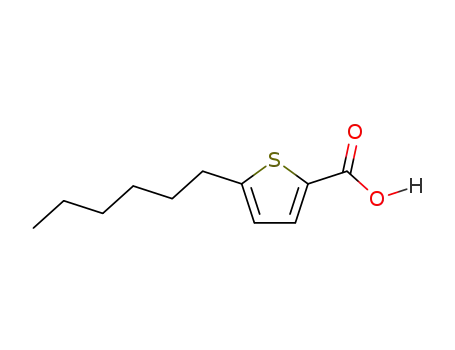
5-Hexyl-2-thiophenecarboxylic acid
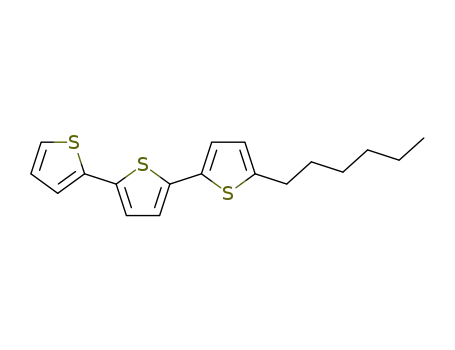
2-hexyl-5-(5-(thiophen-2-yl)thiophen-2-yl)thiophene
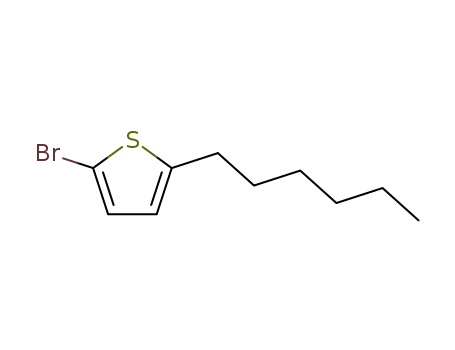
2-bromo-5-hexylthiophene
CAS:51792-34-8
CAS:69249-61-2
CAS:1693-86-3