Your Location:Home >Products >OLED intermediates >Thiophenes >69249-61-2

Product Details
Chemical Properties
Colorless to yellow liquid
Uses
2-Bromo-3-hexylthiophene is used in the synthesis of End-capped Regioregular Poly(3-hexylthiophene). It is also used to synthesize well-defined head-to-tail-type oligothiophenes which are used in a number of high-technology applications including OLEDs.
General Description
2-Bromo-3-hexylthiophene is a monomeric precursor that forms bromo terminate polymers. It is synthesized by the bromination of hexylthiophene.
InChI:InChI=1/C10H15BrS/c1-2-3-4-5-6-9-7-8-12-10(9)11/h7-8H,2-6H2,1H3
Here is reported an expedient synthesis implementing enabling technologies of a family of thiophene-based heptamers alternating electron donor (D) and acceptor (A) units in a D-A′-D-A-D-A′-D sequence. The nature of the peripheral A groups (benzothiadiazole vs. thienopyrrole-dione vs. thiophene-S,S-dioxide) and the strength of the donor units (alkyl vs. thioalkyl substituted thiophene ring) have been varied to finely tune the chemical-physical properties of the D-A oligomers, to affect the packing arrangement in the solid-state as well as to enhance the photovoltaic performances. The optoelectronic properties of all compounds have been studied by means of optical spectroscopy, electrochemistry, and density functional theory calculations. Electrochemical measurements and Kelvin probe force microscopy (KPFM) predicted a bifunctional behaviour for these oligomers, suggesting the possibility of using them as donor materials when blended with PCBM, and as acceptor materials when coupled with P3HT. Investigation of their photovoltaic properties confirmed this unusual characteristic, and it is shown that the performance can be tuned by the different substitution pattern. Furthermore, thanks to their ambivalent character, binary non-fullerene small-molecule organic solar cells with negligible values of HOMO and LUMO offsets were also fabricated, resulting in PCEs ranging between 2.54-3.96%. This journal is
Thiophene-based rings are one of the most widely used building blocks for the synthesis of sulfur-containing molecules. Inspired by the redox diversity of these features in nature, we demonstrate herein a redox-divergent construction of dihydrothiophenes, thiophenes, and bromothiophenes from the respective readily available allylic alcohols, dimethyl sulfoxide (DMSO), and HBr. The redox-divergent selectivity could be manipulated mainly by controlling the dosage of DMSO and HBr. Mechanistic studies suggest that DMSO simultaneously acts as an oxidant and a sulfur donor. The synthetic potentials of the products as platform molecules were also demonstrated by various derivatizations, including the preparation of bioactive and functional molecules.
Semiconducting polymer nanoparticles (SPNs) emitting in the second near-infrared window (NIR-II, 1000-1700 nm) are promising materials for deep-tissue optical imaging in mammals, but the brightness is far from satisfactory. Herein, we developed a molecular design strategy to boost the brightness of NIR-II SPNs: structure planarization and twisting. By integration of the strong absorption coefficient inherited from planar π-conjugated units and high solid-state quantum yield (φPL) from twisted motifs into one polymer, a rise in brightness was obtained. The resulting pNIR-4 with both twisted and planar structure displayed improved φPL and absorption when compared to the planar polymer pNIR-1 and the twisted polymer pNIR-2. Given the emission tail extending into the NIR-IIa region (1300-1400 nm) of the pNIR-4 nanoparticles, NIR-IIa fluorescence imaging of blood vessels with enhanced clarity was observed. Moreover, a pH-responsive poly(β-amino ester) made pNIR-4 specifically accumulate at tumor sites, allowing NIR-IIa fluorescence image-guided cancer precision resection. This study provides a molecular design strategy for developing highly bright fluorophores.
The optoelectronic and structural properties of six stannoles are reported. All revealed extremely weak emission in solution at 295 K, but intensive fluorescence in the solid state with quantum yields (ΦF) of up to 11.1% in the crystal, and of up to 24.4% (ΦF) in the thin film.

3-hexylthiophene

3-hexyl-2-bromothiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetic acid;
at 20 ℃;
for 24h;
|
99% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Darkness;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
for 3h;
Cooling with ice;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 20 ℃;
|
98% |
|
With
N-Bromosuccinimide;
|
98% |
|
With
N-Bromosuccinimide;
In
dichloromethane; acetic acid;
|
98% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
97% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 - 20 ℃;
for 1h;
|
97% |
|
With
N-Bromosuccinimide;
In
acetic acid;
|
96% |
|
With
N-Bromosuccinimide;
In
acetic acid;
Inert atmosphere;
|
96% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 ℃;
|
96% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
|
95% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
for 1h;
cooling;
|
95% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 3h;
|
95% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 20 ℃;
for 0.5h;
|
95% |
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
for 24h;
Inert atmosphere;
|
95% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Inert atmosphere;
|
92% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -10 - 20 ℃;
for 13h;
Inert atmosphere;
Schlenk technique;
Large scale;
|
91% |
|
With
N-Bromosuccinimide; acetic acid;
|
91% |
|
With
N-Bromosuccinimide;
In
acetic acid;
at 0 ℃;
for 2h;
|
89% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 0.5h;
|
88% |
|
With
N-Bromosuccinimide;
In
acetic acid;
for 0.5h;
|
86% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
|
86% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 12h;
|
86% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 - 22 ℃;
for 3.16667h;
|
84% |
|
With
trimethylsilyl bromide; bis-[(trifluoroacetoxy)iodo]benzene;
In
dichloromethane;
at 20 ℃;
|
81% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 20 - 50 ℃;
for 0.5h;
|
79% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
|
78% |
|
With
N-Bromosuccinimide;
In
acetic acid;
at 15 ℃;
for 2.5h;
|
78% |
|
With
N-Bromosuccinimide;
In
acetic acid;
at 22 ℃;
for 2.5h;
|
77% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
for 25h;
Darkness;
|
77% |
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 2h;
Inert atmosphere;
|
70% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 1h;
|
67% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 - 20 ℃;
for 5.5h;
|
52% |
|
With
bromine;
In
acetic acid;
at 0 ℃;
for 0.5h;
|
49% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
Inert atmosphere;
|
47% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: 95 percent / tetrabutylammonium bromide; methanol; Br2 / CH2Cl2 / 20 °C
2.1: ZnBr2; tetrabutylammonium tetrafluoroborate / NiBr2 2,2'-bipyridine / dimethylformamide / -10.16 °C / Electrolysis
2.2: dimethylformamide / Acid hydrolysis
With
methanol; tetrabutylammomium bromide; tetrabutylammonium tetrafluoroborate; bromine; zinc dibromide;
(2,2'-bipyridine)nickel(II) dibromide;
In
dichloromethane; N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 20 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 4h;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
dichloromethane;
at -20 ℃;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 25 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
for 0.5h;
Inert atmosphere;
Darkness;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Cooling with ice;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
Darkness;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane; acetic acid;
for 6h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
|
|
|
With
N-Bromosuccinimide;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 2h;
Inert atmosphere;
|
|
|
With
N-Bromosuccinimide;
In
chloroform;
at -90 - 20 ℃;
for 15h;
|
|
|
With
N-Bromosuccinimide;
at 0 ℃;
|
|
|
With
N-Bromosuccinimide;
|
|
|
With
hydrogen bromide; acetic acid; dimethyl sulfoxide;
at 60 ℃;
for 6h;
Sealed tube;
|
5.1 mg |
2,5-dibromo-3-hexylthiophene


3-hexyl-2-bromothiophene
| Conditions | Yield |
|---|---|
|
2,5-dibromo-3-hexylthiophene;
With
tetrabutylammonium tetrafluoroborate; zinc dibromide;
(2,2'-bipyridine)nickel(II) dibromide;
In
N,N-dimethyl-formamide;
at -10.16 ℃;
Electrolysis;
In
N,N-dimethyl-formamide;
Further stages.;
Acid hydrolysis;
|

3-hexylthiophene

2,5-dibromo-3-hexylthiophene
N,N-dimethyl-formamide
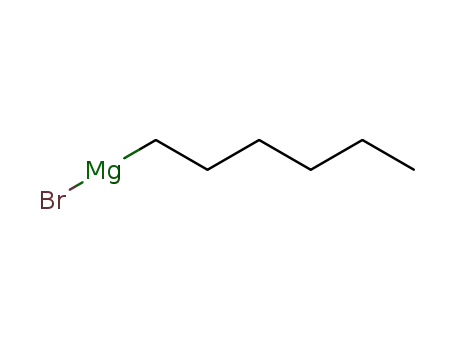
n-hexylmagnesium bromide
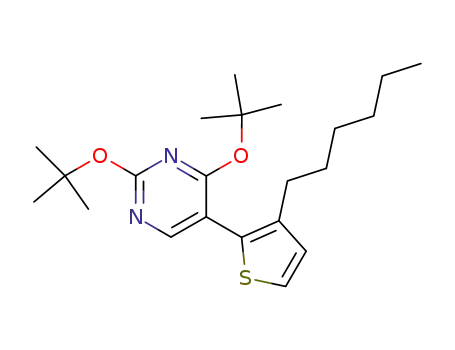
2,4-Di-tert-butoxy-5-(3-hexyl-thiophen-2-yl)-pyrimidine
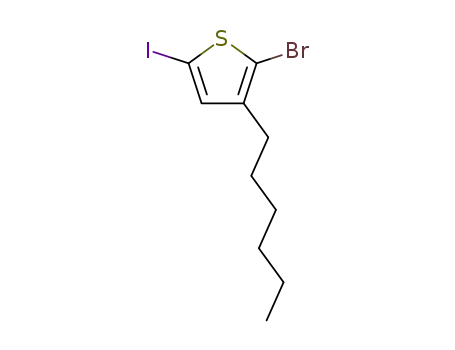
2-bromo-5-iodo-3-hexylthiophene

3-hexylthiophene

3,3'-dihexyl-2,2'-bithiophene
CAS:864377-31-1
CAS:32228-99-2
CAS:116971-11-0
CAS:18794-77-9