Your Location:Home >Products >Organic phosphines >Phenyl phosphines >67292-34-6
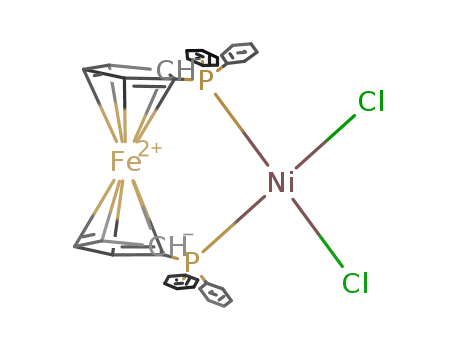

Product Details
Chemical Properties
Yellow powder
Uses
diphosphine 1,1'-bis(diphenylphosphino)ferrocene (dppf) are used in transition metal-catalyzed reactions to synthesize pharmaceuticals and agrochemicals.
Uses
(1,1''-Bis(diphenylphosphino)ferrocene)dichloronickel(II) is an organometallic catalyst used in a variety of synthetic applications, such as the gem-?difluoropropargylation of alkylzinc reagents, or cross coupling reactions to form 2,?3,?5,?6-?tetrasubstituted dehydropiperidines.
Uses
suzuki reaction
The syntheses, electrochemistry, and crystal structures for two new Pt(II) and Pd(II) heteroleptic bimetallic complexes with the crown trithioether 1,4,7-trithiacyclononane (9S3) and the diphosphine ligand, 1,1′-bis(diphenylphosphino)ferrocene (dppf) are reported. Both complexes have the general formula [M(9S3)(dppf)](PF6)2 (M=Pt or Pd) and exhibit the anticipated structure forming a distorted cis square planar array of two sulfur atoms from the 9S3 and two phosphorus atoms. These are, to our knowledge, the first reported examples of dppf transition metal complexes involving a thioether as the ancillary ligand. The dppf ligand functions as a bidentate chelator to a single metal center, and the third 9S3 sulfur atom does interact with the metal ion from a greater distance (Pt-S=2.8167(8) ?; Pd-S=2.7916(5) ?) to yield an elongated square pyramidal geometry. The two structures are isomorphous with very similar bond distances and angles. The values for the 31P-NMR chemical shifts (Pt=15.09 ppm, Pd=-0.47 ppm), the 195Pt-NMR chemical shift for the Pt(II) complex (-4353 ppm) and 1J(195Pt-31P) coupling constants (3511 Hz) are all consistent with a cis-MS2P2 square planar coordination sphere. The 9S3 ligand is fluxional in solution for both complexes. The electrochemistry of both complexes is dominated by a reversible Fe(II)/Fe(III) couple from the ferrocene moiety (E1/2=+721 mV for Pt(II), +732 mV for Pd(II), both versus Fc/Fc+).
The catalytic transfer hydrogenation reactions of a series of aromatic and aliphatic carbonyl compounds were investigated using divalent Ni(II)-diphosphine complexes, [L2NiCl2] (where L2 = 1,1-bis(diphenylphosphino)methane (dppm), 1,2-bis(diphenylphosphino)ethane (dppe), 1,3-bis(diphenylphosphino)propane (dppp), 1,1-bis(diphenylphosphino)ferrocene (dppf), and N-butyl-N-(diphenylphosphino)-1,1-diphenylphosphinamine (dppba)). This is a single-step reaction in the presence of potassium hydroxide and isopropyl alcohol to afford the corresponding alcohols. This protocol tolerates other sensitive functional groups like olefinic double bonds and also achieves high chemoselectivity. All the reactions were monitored by GC and GC–MS. The plausible mechanism is also discussed. The method reported in the present article is simple, cost-effective, and provides excellent conversions. Nickel-diphosphine complexes appear as a potential alternative to expensive transition metal complexes.
The invention discloses a method for the preparation of fluoro alkylated compounds by homogeneous Ni catalyzed fluoro alkylation with fluoro alkyl halides in the presence of a base.
The invention discloses a method for the preparation of fluoro alkylated 1,4-dioxene by homogene- ous Ni catalyzed fluoro alkylation with fluoro alkyl halides of 1,4-dioxane in the presence of a base.
This communication describes the synthesis of a series of diphosphine NiII(Ph)(CF3) complexes and studies of their reactivity toward oxidatively induced Ph-CF3 bond-forming reductive elimination. Treatment of these complexes with the one-electron outer-sphere oxidant ferrocenium hexafluorophosphate (FcPF6) affords benzotrifluoride, but the yield varies dramatically as a function of diphosphine ligand. Diphosphines with bite angles of less than 92° afforded 3. In contrast, those with bite angles between 95 and 102° formed PhCF3 in yields ranging from 62 to 77%.

1,1'-bis-(diphenylphosphino)ferrocene

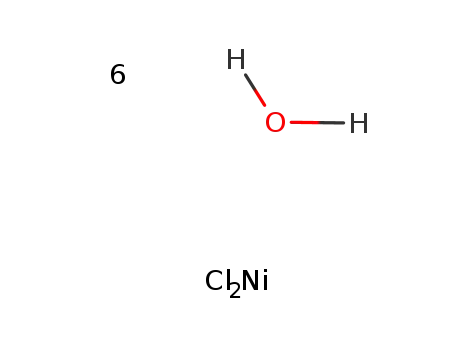
nickel(II) chloride hexahydrate

![[1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride](/upload/2023/2/c8099d24-38a9-426d-a76a-a275743c4b2c.png)
[1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride
| Conditions | Yield |
|---|---|
|
In
ethanol;
at 80 ℃;
for 0.5h;
Sealed tube;
Inert atmosphere;
|
97% |
|
In
dichloromethane;
at 20 ℃;
for 20h;
Inert atmosphere;
|
91% |
|
In
ethanol;
at 80 ℃;
for 0.5h;
Inert atmosphere;
|
89% |
|
In
ethanol;
at 80 ℃;
for 0.5h;
Inert atmosphere;
Schlenk technique;
|
85% |
|
In
ethanol;
at 0 - 80 ℃;
for 0.666667h;
Inert atmosphere;
Schlenk technique;
|
85% |
|
In
ethanol;
at 80 ℃;
for 0.5h;
|
85% |
|
In
methanol; isopropyl alcohol;
NiCl2*6H2O was heated to boiling in 2-propanol/MeOH, soln. of Fe-complexin hot 2-propanol was added, refluxed for 30 min, cooled; filtered, washed with cold MeOH and ether;
|
60.7% |
|
In
methanol;
for 24h;
Inert atmosphere;
Reflux;
|
C41H32F4FeNiP2


lithium chloride

![[1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride](/upload/2023/2/c8099d24-38a9-426d-a76a-a275743c4b2c.png)
[1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride

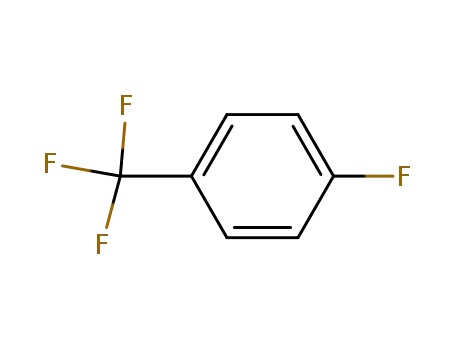
4-Fluorobenzotrifluoride
| Conditions | Yield |
|---|---|
|
With
ferrocenium hexafluorophosphate;
In
acetone;
at 23 ℃;
for 0.5h;
Inert atmosphere;
Glovebox;
|
72 %Spectr. |

1,1'-bis-(diphenylphosphino)ferrocene
nickel dichloride
bis(triphenylphosphine)nickel(II) chloride
carbon monoxide
(1,1′-bis(diphenylphosphino)ferrocene)2Ni
[(1,1′-bis(diphenylphosphino)ferrocene)Ni(0)(1,5-cyclooctadiene)]
[Ni(S2CN(CH2CH=CH2)2)(1,1'-bis(diphenylphosphino)ferrocene)]PF6
cis-[1,1'-bis(diphenylphosphino)ferrocene](2-methylphenyl)nickel(II) chloride
CAS:125143-53-5
CAS:333432-28-3
Molecular Formula:C15H15BO2
Molecular Weight:238.09
CAS:15629-92-2
Molecular Formula:C27H26Cl2NiP2
Molecular Weight:542
CAS:14264-16-5