Your Location:Home >Products >Organic phosphines >Phenyl phosphines >14264-16-5
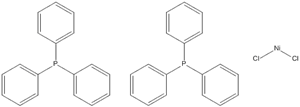

Product Details
Reaction
Catalyst for hydrosilylation of styrene with diphenysilane Catalyst for carboxylation of various aryl chlorides and other derivatives Catalyst for C–P cross-coupling reactions of diphenylphosphine oxide with aryl chloride Catalyst for N?Heterocyclic carbene-assisted cross-coupling reactions of diarylborinic acids with aryl chlorides,tosylates, and sulfamates. Catalyst for Negishi biaryl ketone synthesis by cross-coupling of amides with aryl zinc halides via carbon-nitrogen bond cleavage.
Chemical Properties
dark green to dark grey crystals or powder
Uses
Coordination compund.
Uses
suzuki reaction
Uses
Dichlorobis(triphenylphosphine)nickel(II) is used as a catalyst for cross-coupling of Grignard reagents, hydrosilylations, hydrogenation and polymerization.
Purification Methods
Wash it with glacial AcOH and dry it in a vacuum over H2SO4 and KOH until AcOH is removed. [Venanzi J Chem Soc 719 1958, Kocienski et al. J Org Chem 54 1215 1989, Beilstein 16 IV 953.]
InChI:InChI=1/2C18H15P.2ClH.Ni/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2
A computational screening of 42 bidentate phosphines (PP) has yielded promising candidates for Ph-CF3 reductive elimination from Ni(II) complexes of the type [(PP)Ni(Ph)(CF3)]. The computed barriers and synthetic accessibility consid
Abstract: New three-component reaction was developed via one-pot strategy for the synthesis of functionalized 3,3′-disubstituted oxindoles and spirooxindole through the reaction among isatin, malononitrile, and acetone/indole/nitromethane/acetylacetone/di
Sulphonamides and carboxamides have great pharmacological importance. The purpose of the study was to synthesize alanine-derived bioactive sulphonamides bearing carboxamides and evaluate their biological activi-ties. The reaction of p-toluenesulphonyl chloride with L-alanine afforded compound 1, which was acetylated to obtain compound 2. The chlorina-tion and ammonolysis of compound 2 gave the carboxamide backbone (3) which was coupled with aryl/heteroaryl halides to afford the hybrid compounds 4, 5 and 6. Structures were confirmed by FTIR,1 H-NMR,13 C-NMR spectra and elemental analytical data. The in vitro antimicrobial properties were determined by agar dilution, and the antioxidant properties were also investigated. Molecular docking interactions of the analogues were determined using PyRx. Compounds 4, 5 and 6 exhibited excellent in vitro antimi-crobial properties in the range of 0.5-1.0mg/ml while compounds 1and 2 had half-maximal inhibitory concentration (IC50) of 1.11±0.15μg/ml and 1.12±0.13μg/ml respectively. For the molecular docking studies, compounds 5 and 6 displayed the best antitrypanosomal activity with binding affinities of-13.95 and-13.51kcal/mol respectively while compound 4 showed the highest in silico antimalarial activity having binding affinity of-11.95kcal/mol. All the alanine derived sulphonamides were observed to be potential antimicrobial, antioxidant, antitrypanosomal and antimalarial agents following the biological activities studies.
Both Ni(0) and Ni(I) compounds are believed to exhibit cross-coupling catalytic properties under various conditions, and the compounds Ni(PPh3)4 and NiCl(PPh3)3 are compared as catalysts for representative Suzuki-Miyaura and Heck-Mizoroki cross-coupling reactions. The Ni(0) compound exhibits catalytic activities, for cross-coupling of chloro and bromoanisole with phenylboronic acid and of bromobenzene with styrene, yielding results which are comparable with those of many palladium-based catalysts, but our findings with NiCl(PPh3)3 are at this point unclear. It seems to convert to catalytically active Ni(0) species under Suzuki-Miyaura reaction conditions and is ineffective for Heck-Mizoroki cross-coupling. The paramagnetic Ni(I) compounds NiX(PPh3)3 (X = Cl, Br, I) are characterized for the first time by 1H NMR spectroscopy and are found to exhibit broad meta and para resonances at δ 9-11 and 3-4, respectively, and very broad ortho resonances at δ 46; these resonances are very useful for detecting Ni(I) species in solution. The chemical shifts of NiCl(PPh3)3 vary with the concentration of free PPh3, with which it exchanges, and are also temperature-dependent, consistent with Curie law behavior. The compound trans-NiPhCl(PPh3)2, the product of oxidative addition of chlorobenzene to Ni(PPh3)4 and a putative intermediate in cross-coupling reactions of chlorobenzene, is found during the course of this investigation to exhibit entirely unanticipated thermal lability in solution in the absence of free PPh3. It readily decomposes to biphenyl and NiCl(PPh3)2 in a reaction relevant to the long-known but little-understood nickel-catalyzed conversion of aryl halides to biaryls. Ni(I) and biphenyl formation is initiated by PPh3 dissociation from trans-NiPhCl(PPh3)2 and formation of a dinuclear intermediate, a process which is now better defined using DFT methodologies.
The invention discloses a synthesis method of eletriptan hydrobromate, which comprises the following steps: reacting (R)-1-acetyl-3-(N-methylpyrrolidinyl-2-methy)-5-bromo-1H-indole and metal in an organic solvent to form a metal complex, reacting the metal complex with a boron reagent in an organic solvent to form aryl borane or aryl borate, carrying out acid-catalyzed hydrolysis to obtain aryl boric acid, and carrying out coupling and hydrolysis on the aryl boric acid and 2-chloroethylphenyl sulfone under the actions of a catalyst and an alkali to obtain eletriptan, or directly carrying out coupling and hydrolysis on the metal complex and 2-chloroethylphenyl sulfone to obtain eletriptan; and carrying out salification on the eletriptan and hydrobromic acid in an organic solvent to finally obtain the eletriptan hydrobromate. The method is simple to operate, has the advantages of high safety, high stability, low cost and higher yield, and is suitable for industrial production.
[((C6H5)3P)3NiCl]2

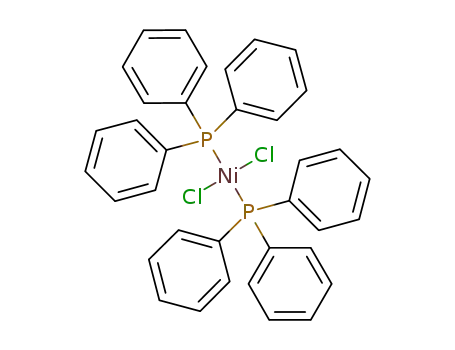
bis(triphenylphosphine)nickel(II) chloride


nickel

nickel dichloride
| Conditions | Yield |
|---|---|
|
In
neat (no solvent);
byproducts: triphenylphosphine; Ar-atmosphere; 140-180°C, 1 h; extn. (ether, CH2Cl2, H2O), evapn. of extract;
|
97.7% 87.5% 94.7% |
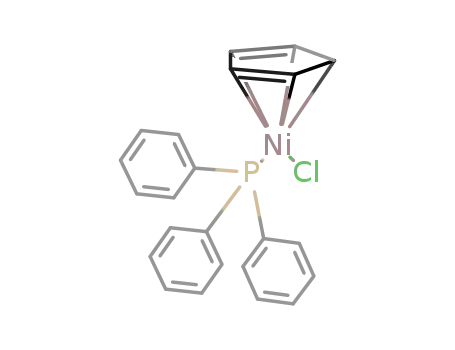
chloro(cyclopentadienyl)(triphenylphosphine)nickel(II)


bis(triphenylphosphine)nickel(II) chloride

nickelocene
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
dispropn.; 25°C;
|
|
|
In
1,2-dimethoxyethane;
25°, 10E-3 molar soln.;
|
|
|
In
1,2-dimethoxyethane;
|
|
|
In
acetonitrile;
|
triphenylphosphine

nickel dichloride
chloroethylene
(6-phenyl-2-((2,6-diisopropylphenyl)iminomethyl)phenoxide)Ni(Ph)(PPh3)
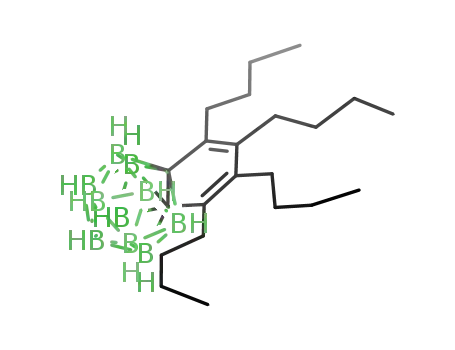
C2B10H10(C4(C4H9)4)
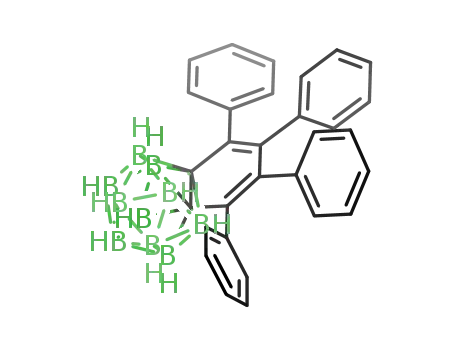
C2B10H10(C4(C6H5)4)
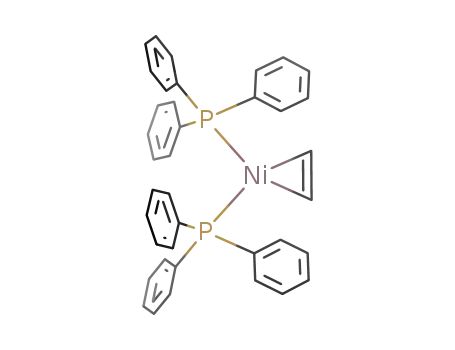
bis(triphenylphosphine)ethylenenickel(0)
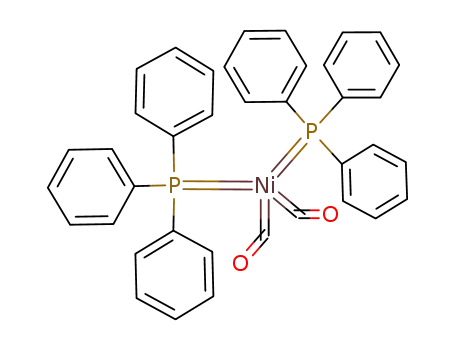
bis(triphenylphosphine)nickel(0) dicarbonyl
CAS:781-73-7
CAS:67292-34-6
CAS:14126-37-5