Your Location:Home >Products >Organic phosphines >Phenyl phosphines >14126-37-5
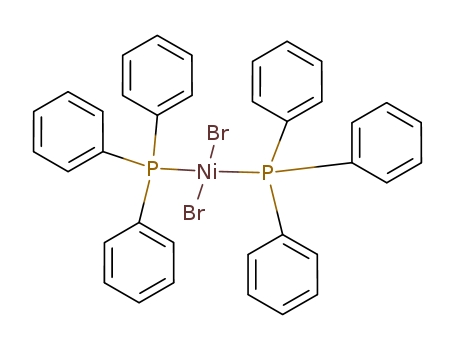

Product Details
Chemical Properties
DARK GREEN CRYSTALLINE POWDER OR CRYSTALS
Uses
As a polymerization catalyst
Uses
Catalysts for the cross-coupling reactions, C-X bond reduction, homocoupling of Csp2 halides, displacement of aryl halides, and oligomerization of dienes
InChI:InChI=1/2C18H15P.2BrH.Ni/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2
Both Ni(0) and Ni(I) compounds are believed to exhibit cross-coupling catalytic properties under various conditions, and the compounds Ni(PPh3)4 and NiCl(PPh3)3 are compared as catalysts for representative Suzuki-Miyaura and Heck-Mizoroki cross-coupling reactions. The Ni(0) compound exhibits catalytic activities, for cross-coupling of chloro and bromoanisole with phenylboronic acid and of bromobenzene with styrene, yielding results which are comparable with those of many palladium-based catalysts, but our findings with NiCl(PPh3)3 are at this point unclear. It seems to convert to catalytically active Ni(0) species under Suzuki-Miyaura reaction conditions and is ineffective for Heck-Mizoroki cross-coupling. The paramagnetic Ni(I) compounds NiX(PPh3)3 (X = Cl, Br, I) are characterized for the first time by 1H NMR spectroscopy and are found to exhibit broad meta and para resonances at δ 9-11 and 3-4, respectively, and very broad ortho resonances at δ 46; these resonances are very useful for detecting Ni(I) species in solution. The chemical shifts of NiCl(PPh3)3 vary with the concentration of free PPh3, with which it exchanges, and are also temperature-dependent, consistent with Curie law behavior. The compound trans-NiPhCl(PPh3)2, the product of oxidative addition of chlorobenzene to Ni(PPh3)4 and a putative intermediate in cross-coupling reactions of chlorobenzene, is found during the course of this investigation to exhibit entirely unanticipated thermal lability in solution in the absence of free PPh3. It readily decomposes to biphenyl and NiCl(PPh3)2 in a reaction relevant to the long-known but little-understood nickel-catalyzed conversion of aryl halides to biaryls. Ni(I) and biphenyl formation is initiated by PPh3 dissociation from trans-NiPhCl(PPh3)2 and formation of a dinuclear intermediate, a process which is now better defined using DFT methodologies.
The activity of the catalytic system NiBr2(PPh3) 2/Zn/PhI in polymerization of butyl acrylate and butyl methacrylate and in copolymerization of butyl methacrylate with styrene was examined.
The present invention relates to linear pyridazine compounds, and more particularly to those of these compounds which are oligopyridazine compounds, to processes for obtaining them, to their uses, and also to their reduction to pyrroles and to the uses of the pyrrole, pyridazinylpyrrole and oligopyrrole compounds obtained. The invention relates in particular to the uses as medicaments, in particular for treating pathologies such as cancer, bacterial infections or parasitic infections, and also the applications in the materials, environmental, electronics and optics field.
2-(N-aryliminomethyl)pyrrole precursors (2,6-R2-C6H3-N{double bond, long}CH-2-C4H3NH) (R = Me, IH; R = iPr, IIH) were prepared and transformed into their corresponding sodium salts (Na+I- and Na+II-) by treatment with NaH. Both salts readily react with [NiBr2(DME)] (DME = 1,2-dimethoxyethane) to give the respective bis{2-(N-arylimino-κN-methyl)pyrrolide-κN}nickel(II) complexes (1, 2) in almost quantitative yields. The oxidative addition of IH to [Ni(COD)2] (COD = 1,5-cyclooctadiene) results in the formation of 3, which is a mono(iminomethylpyrrolide)-η3-(cyclic-allyl)-type organonickel(II) complex. The crystal structure of compound 1 has been established by X-ray diffraction studies.
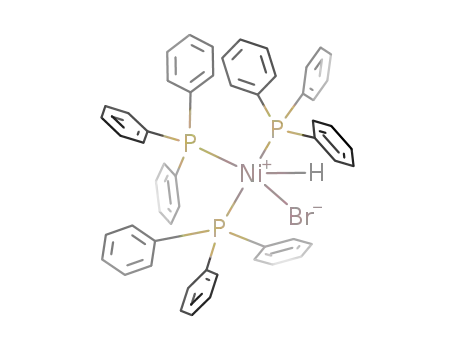
((C6H5)3P)3Ni(H)Br

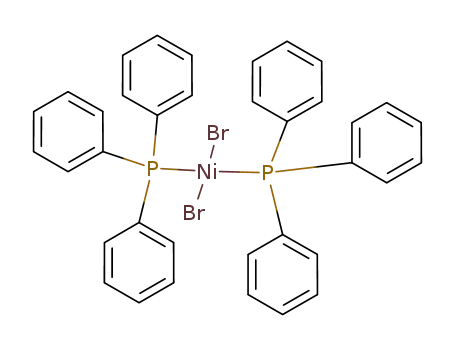
dibromobis(triphenylphosphine)nickel(II)

bis(triphenylphosphine)nickel(0) dicarbonyl
| Conditions | Yield |
|---|---|
|
With
carbon monoxide;
In
tetrahydrofuran;
byproducts: (C6H5)3P; (Ar), to nickel complex added THF previously saturated with CO, CO passed in for 2.5 h at -20°C, pptd.; ppt. after washing (THF, pentane) obtained (Ph3P)2NiBr2, filtrate evaptd., washed (pentane) extracted (ether), chromy (Al2O3, benzene/petroleumether 1:1), elem. anal., IR;
|
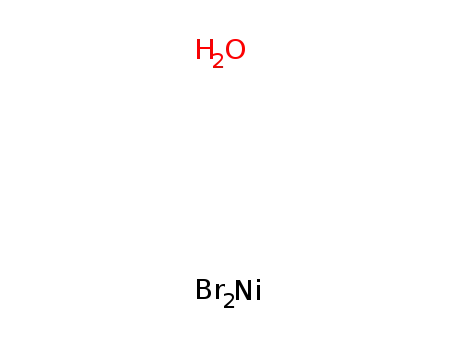
nickel(II) bromide monohydrate

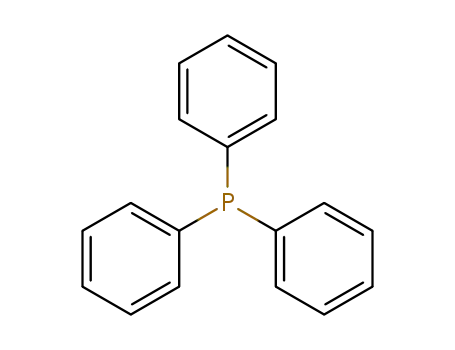
triphenylphosphine

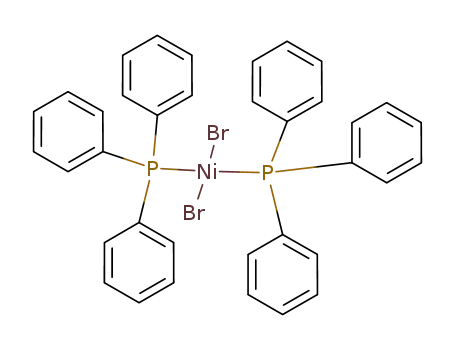
dibromobis(triphenylphosphine)nickel(II)
| Conditions | Yield |
|---|---|
|
In
butan-1-ol;
at 20 ℃;
Reflux;
|
60% |

((C6H5)3P)3Ni(H)Br
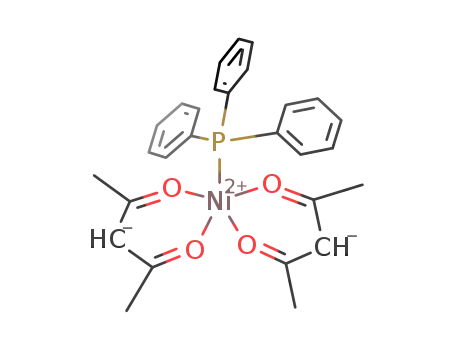
Ni(acetylacetonate)2(triphenylphosphine)
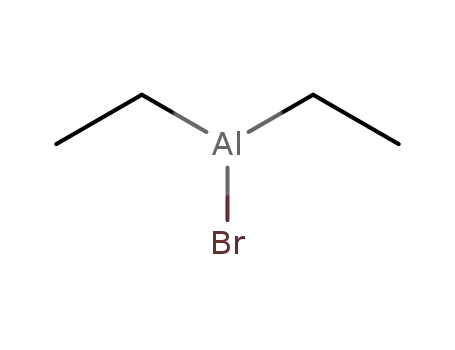
diethylaluminum bromide
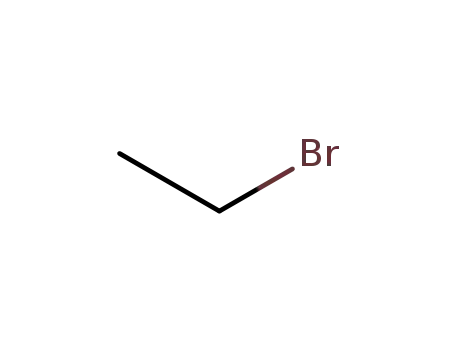
ethyl bromide

nickel
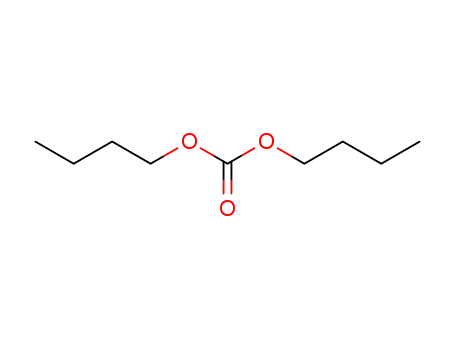
Dibutyl carbonate
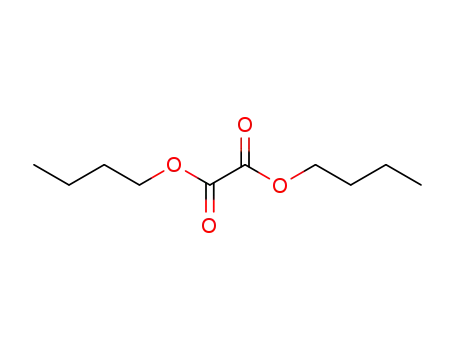
di-n-butyl oxalate
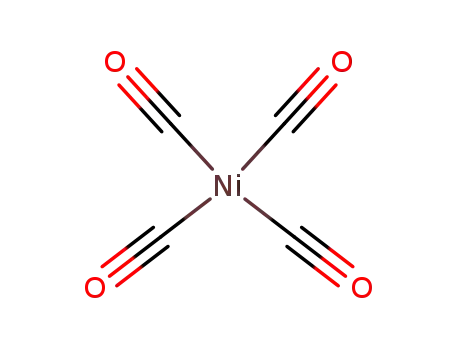
tetracarbonyl nickel
CAS:139100-06-4
CAS:14264-16-5
CAS:16903-61-0