Your Location:Home >Products >Functional intermediates >102113-98-4
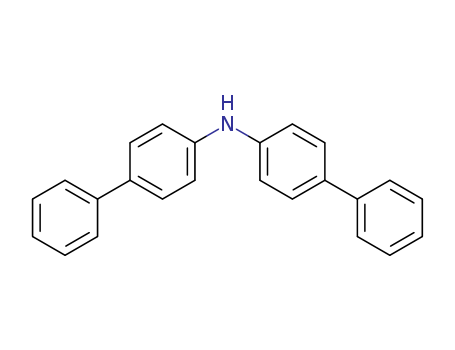

Product Details
Uses
Bis(4-biphenylyl)amine can be used as organic synthesis intermediate and pharmaceutical intermediate, mainly in laboratory research and development process and chemical production process.
Synthesis
Bis(4-bromophenyl)amine (4.0 g, 12.3 mmol) and phenylboronic acid (4.0 g, 32.7 mmol) were mixed in 250 mL of toluene and 60 mL of ethanol. The solution was bubbled with nitrogen while stirring for 15 minutes. Pd(PPh3)4 (1.4 g, 1.23 mmol) and K3PO4 (13.5 g, 64 mmol) were added in sequence. The mixture was heated to reflux overnight under nitrogen. After cooling, the reaction mixture was filtered through filter paper and the solvent was then evaporated. The solid was redissolved in nitrogen-purged hot toluene and was filtered through a Celite?/silica pad when the solution was still hot. The solvent was then evaporated. The white crystalline solid was washed by hexane and air dried to obtain 3.8 g of Bis(4-biphenylyl)amine.
We report herein on the results of our combined experimental/computational study regarding the catalytic performance of PAd-DalPhos (L1) in nickel-catalyzed ammonia arylation for primary aniline synthesis. Primary arylamine C-N reductive eliminations occurring from arylnickel(II) parent amido complexes of the type (L)Ni(Ph)(NH2) were modeled by use of density-functional theory (DFT) methods, for a series of L1 derivatives. The dual aims were to assess the effect of structural modifications to L1 on potentially rate-limiting C-N reductive elimination and to identify promising candidates for experimental inquiry. Increasing the steric demand of the Paryl groups from o-tolyl (in L1) to mesityl (in L16) resulted in a significant lowering of the barrier to C-N reductive elimination (ΔG?RE), which can be attributed in part to interactions between the ligand Paryl groups and the nickel-bound amido ligand, as observed in noncovalent interaction (NCI) plots of the reductive elimination transition-state structures. Despite the favorability of L16 predicted on the basis of computational analysis focusing on C-N reductive elimination, this ancillary ligand performed poorly in experimental testing versus L1, suggesting that in practice the significant steric demands of L16 may discourage the formation of key catalytic intermediates. Modifications to the steric profile of the Paryl groups in L1 led to dramatic changes in catalytic performance, with the presence of an o-methyl proving to be important, among the L1 variants tested, in achieving useful catalytic performance in the Ni-catalyzed monoarylation of ammonia.
We synthesized four hyper-conjugated aromatic hole transporting materials with different molecular geometry and energy levels by attaching arylamino moiety attached to the carbazole core. A brominated carbazole moiety reacted with an arylamino moiety using a Buchwald-Hartwig reaction. The characteristics of these hole transporting materials were investigated using TGA, DSC, UV–Vis and luminescence spectroscopy. The energy levels of all materials used in this study were estimated from cyclic voltammograms and absorption spectra. The hole transporting properties of the synthesized molecules were measured using single-carrier devices. All four hole transporting materials showed similar hole mobility. The effectiveness of hole transporting materials was compared by fabricating green-emitting organic light emitting diode (OLED) devices. It turned out that the device performances were critically dependent on the relative energy levels of the hole transporting layer and emission layer. However, the molecular geometry greatly influenced the device lifetime, determining thermally induced crystallization by the heat produced during device operation.
The invention provides a preparation method of di (4-biphenyl) amine, which comprises the following steps: synthesizing an intermediate di (4-biphenyl) acetamide, and removing acetyl of the intermediate to obtain a target product. The preparation method comprises the following steps: 1) mixing 4-bromobiphenyl, acetamide, a catalyst, a nitrogen-containing ligand L, alkali 1 and a solvent 1, and heating to react; 2) after the reaction, cooling the reaction system to room temperature, washing with water, separating liquid, and concentrating an organic phase to obtain a crude product of bis (4-biphenyl) acetamide; 3) loading the di (4-biphenyl) acetamide crude product with column chromatography silicon dioxide, and purifying with a column chromatography separation and purification method to obtain di (4-biphenyl) acetamide; 4) mixing di (4-biphenyl) acetamide, alkali 2 and a solvent 2, and heating to react; and 5) after the reaction, cooling the reaction system to room temperature, and carrying out suction filtration, water washing and drying to obtain di (4-biphenyl) amine. The invention belongs to the field of fine chemical engineering, and the preparation method is economical, safe, environment-friendly, efficient and suitable for industrial production.
A mixture DMSO-allyl bromide has been developed as a reagent for an atom economic one-pot N-allylation and aryl bromination under basic conditions. Utilizing this reagent, N-allylation-bromination of a number of 2°-aryl amines, aryl aminoamides, indoles, and 7-aza indoles has been achieved. The scope of the substrates and limitations, the synthetic utility of the products, and a plausible reaction mechanism have been proposed.
The invention relates to the technical field of organic electroluminescent materials, in particular to an organic electroluminescent material 9 with 10 -9 dihydro 9 -10 -dimethyl and oxanthrene and arylamine groups, an electronic device containing the compound and a device. The organic electroluminescent device has lower driving voltage. Higher luminous efficiency and longer service life.

N,N-di-(4-biphenyl)benzylamine

palladium-active carbon


palladium

![N,N-di([1,1′-biphenyl]-4-yl)-4′-bromo-[1,1′-biphenyl]-4-amine](/upload/2023/2/0553aa0f-2493-4a86-92d2-39ab78a0e6fe.png)
N,N-di([1,1′-biphenyl]-4-yl)-4′-bromo-[1,1′-biphenyl]-4-amine

bis(biphenyl-4-yl)amine
| Conditions | Yield |
|---|---|
|
With
hydrogen;
In
ethanol; dichloromethane; chloroform; toluene;
|
4-bromo-1,1'-biphenyl


bis(biphenyl-4-yl)amine

4-phenylalanine
| Conditions | Yield |
|---|---|
|
With
tris(dibenzylideneacetone)dipalladium (0); Ti-N complexes; (S)-(1,1'-binaphthalene)-2,2'-diylbis(diphenylphosphine); sodium t-butanolate;
In
toluene;
at 90 ℃;
for 40h;
|
4% 39% |
|
With
tris(dibenzylideneacetone)dipalladium (0); Ti-N complexes; tris-(o-tolyl)phosphine; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 4h;
Product distribution;
Mechanism;
other aryl bromides and triflates; other ligands and solvent; var. temp. and reaction time;
|
32% 11% |
|
With
tris(dibenzylideneacetone)dipalladium (0); Ti-N complexes; tris-(o-tolyl)phosphine; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 40h;
|
23% 28% |
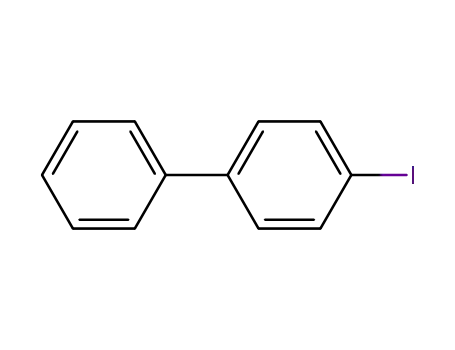
4-iodo-biphenyl
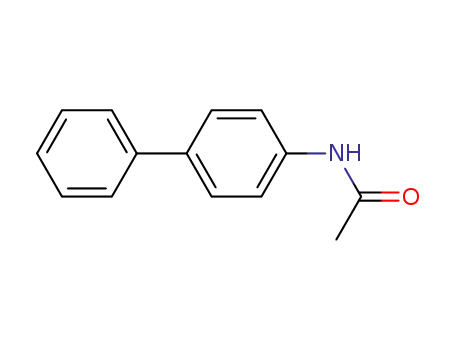
4-phenylacetanilide

4-phenylalanine
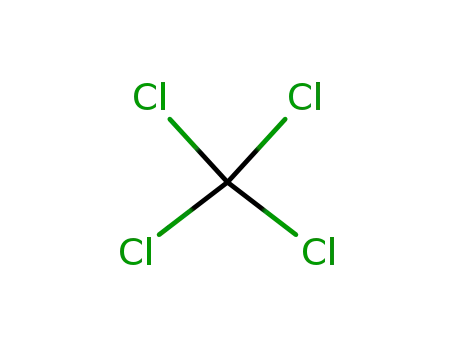
tetrachloromethane

N-([1,1'-biphenyl]-4-yl)-N-phenyl-[1,1'-biphenyl]-4-amine

tris(p-biphenyl)amine
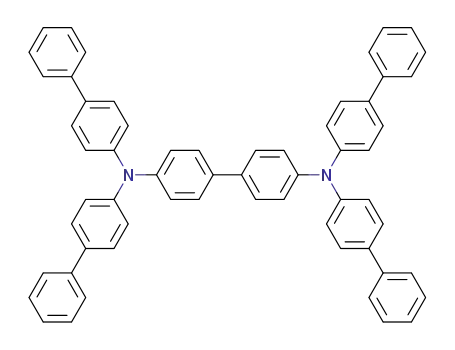
N,N,N',N'-tetra[(1,1'-biphenyl)-4-yl]-(1,1'-biphenyl)-4,4'-diamine
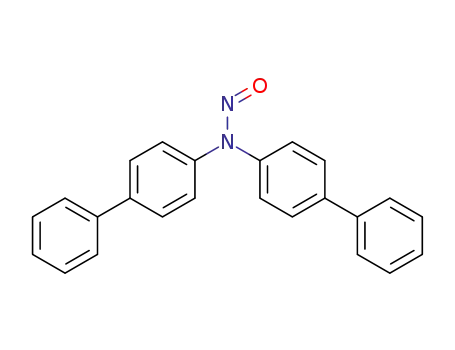
bis-biphenyl-4-yl-nitroso-amine
CAS:932710-63-9
Molecular Formula:C16H28NP
Molecular Weight:265.37
CAS:116971-10-9
CAS:32228-99-2
CAS:18666-26-7