Your Location:Home >Products >Functional intermediates >32228-99-2
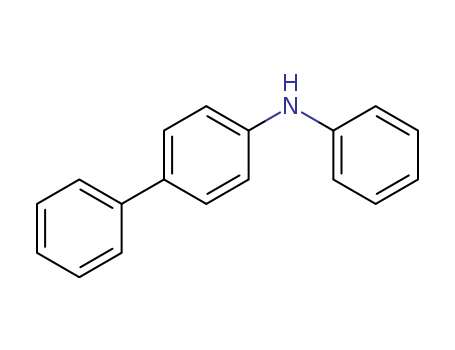

Product Details
Uses
N-Phenyl-4-biphenylamine (cas# 32228-99-2) is a useful reagent for the preparation of heterocyclic compounds for organic electrical devices.
InChI:InChI=1/C18H15N/c1-3-7-15(8-4-1)16-11-13-18(14-12-16)19-17-9-5-2-6-10-17/h1-14,19H
The invention relates to the technical field of organic electroluminescent materials, in particular to an organic electroluminescent material 9 with 10 -9 dihydro 9 -10 -dimethyl and oxanthrene and arylamine groups, an electronic device containing the compound and a device. The organic electroluminescent device has lower driving voltage. Higher luminous efficiency and longer service life.
The invention relates to an organic compound, an electronic device comprising the organic compound, and electronic equipment comprising the electronic device. The structural formula of the organic compound is represented by a chemical formula 1, and the organic compound is applied to the electronic device and can significantly improve the performance of the electronic device.
The invention provides an organic compound and an electronic element and an electronic device comprising the same, and belongs to the technical field of organic electroluminescence. The structural formula of the organic compound is composed of a structure as shown in a chemical formula 1, and the organic compound has excellent photoelectric properties, can improve the luminous efficiency and the service life of the device, and can reduce the working voltage.
The invention relates to an organic compound. The structure of the organic compound is shown as a formula I. When the organic compound is used as a hole adjustment layer material of an electronic element, driving voltage can be reduced, the luminous efficiency of a device can be improved, and the service life of the device can be prolonged.
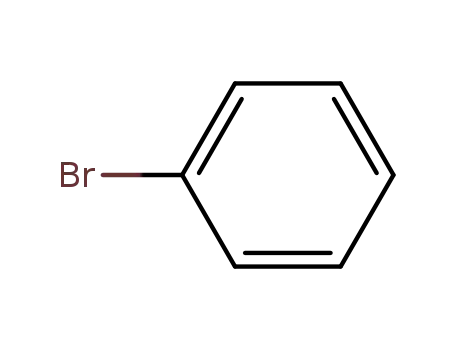
bromobenzene

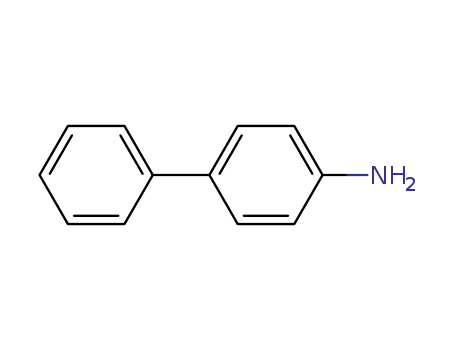
4-phenylalanine

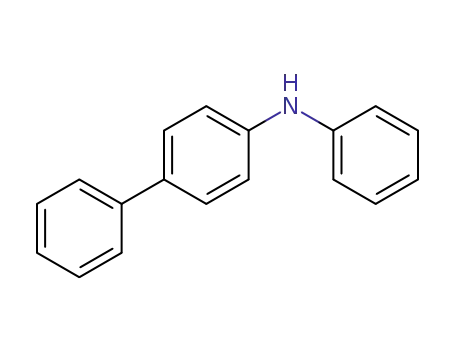
4-phenyldiphenylamine
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 108 ℃;
for 2h;
Inert atmosphere;
|
86% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 108 ℃;
for 2h;
Inert atmosphere;
|
86% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
at 80 ℃;
|
75% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
for 1h;
Inert atmosphere;
Reflux;
|
75% |
|
With
tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate;
In
toluene;
at 110 ℃;
Inert atmosphere;
|
73% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
for 5h;
Inert atmosphere;
Reflux;
|
65% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; tri tert-butylphosphoniumtetrafluoroborate;
In
toluene;
at 100 ℃;
for 7h;
Inert atmosphere;
|
65% |
|
With
potassium phosphate; XPhos;
tris-(dibenzylideneacetone)dipalladium(0);
In
toluene;
for 20h;
Heating;
|
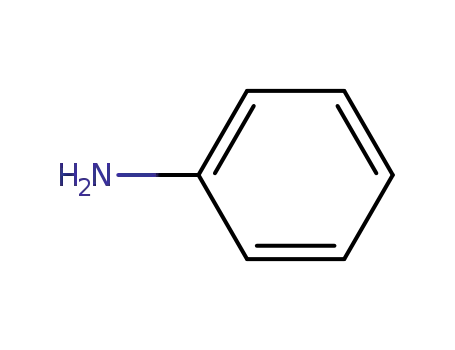
aniline

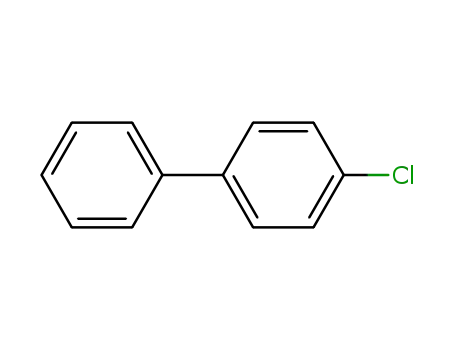
4'-biphenyl chloride


4-phenyldiphenylamine
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 70 ℃;
for 1h;
Inert atmosphere;
|
89.3% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 65 - 75 ℃;
for 1h;
Inert atmosphere;
|
86.9% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos;
In
toluene;
at 20 - 70 ℃;
for 1h;
Inert atmosphere;
|
86.9% |
|
aniline; 4'-biphenyl chloride;
With
palladium diacetate; sodium sulfate; sodium t-butanolate; XPhos;
for 1h;
Milling;
In
water; ethyl acetate;
for 0.0333333h;
Milling;
|
62% |
|
With
[tert-butyloxy]bis(N,N-diisopropylamino)phosphane; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate;
In
toluene;
at 105 ℃;
for 24h;
Inert atmosphere;
Schlenk technique;
|
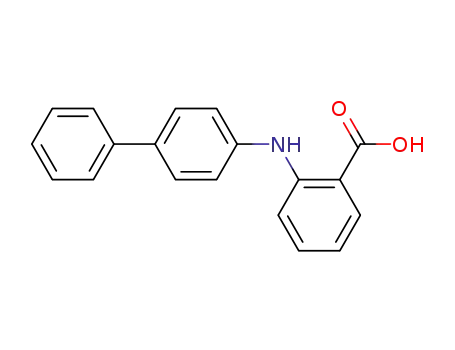
2-[(4-biphenyl)amino]benzoic acid
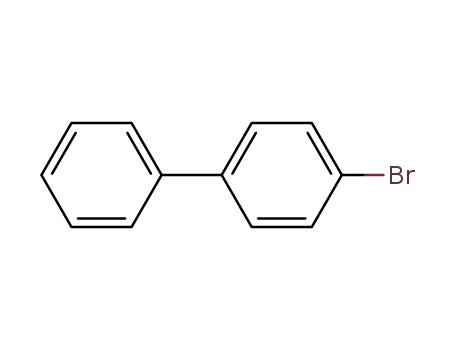
4-bromo-1,1'-biphenyl
Acetanilid
biphenyl
3-phenyl-9H-carbazole
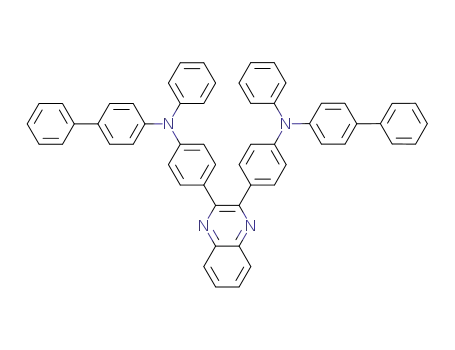
2,3-bis{4-[N-(4-biphenylyl)-N-phenylamino]phenyl}quinoxaline

C55H40N4O

C52H38N2
CAS:11070-44-3
Molecular Formula:C<sub>9</sub>H<sub>10</sub>O<sub>3</sub>
Molecular Weight:166.17
CAS:894791-46-9
Molecular Formula:C24H16BrN
Molecular Weight:398.3
CAS:1448787-63-0
CAS:102113-98-4