
Product Details
Chemical Properties
WHITE CRYSTALLINE POWDER
Uses
Carbic Anhydride is an intermediate used to prepare Endo-2,3-Norbornanedicarboximide (N661225) which is used to prepare piperidinylbenzisoxazole antipsychotic drugs.
Purification Methods
-methanoisobenzofuran-1,3-dione) [129-64-6] M 164.2, m 164.1o, 164 -1 6 5o, 165-167o, d 4 1.417. It forms crystals from pet ether, hexane or cyclohexane. It is hydrolysed by H2O to form the acid [Diels & Alder Justus Liebigs Ann Chem 460 98 1928, Maitte B
InChI:InChI=1/C9H8O3/c10-8-6-4-1-2-5(3-4)7(6)9(11)12-8/h1-2,4-7H,3H2/t4-,5+,6?,7?
Two novel compounds [Zn2(Endc)2(bipy)2(H2O)3]·4(H2O)·2(O)(1), [Zn2(Endc)2(phen)2(H2O)]·(O)(2) (bipy Combining double low line 2,2-bipyridine, phen Combining double low line 1,10-phenanthroline, and Endc Combining double low line endo-norbornene-cis-5,6-dicarboxylicacid) have been synthesized and characterized. In this paper abbreviations are FS-DNA (fish sperm DNA), HeLa (human cervix epithelia carcinoma cells), KB (human oral epithelial carcinoma cells), LO2 (human liver cell L-O2), EtBr (ethidium bromide), DMF (Dimethyl Formamide), MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium]). The binding of complexes with Fish Sperm DNA were measured by electronic absorption spectra and fluorescence spectroscopy. The ability of these complexes to cleave the pBR322 plasmid DNA or the KB and HeLa DNA extracted in our laboratory was demonstrated by gel electrophoresis assay. The cytotoxic effects of these complexes were examined on two tumor cell lines, HeLa, KBr and one normal cell line LO-2. UV absorption and fluorescence spectra indicate the ability of the complexes bond to DNA with different binding affinity. Gel electrophoresis assay demonstrates which one complex more effective DNA-cleavage activity. The cytotoxic activity of the complexes was tested against two different cancer and one normal cell lines. The two complexes exhibited cytotoxic specificity and significant cancer cell inhibitory rate and lower cytotoxicity toward the normal cell lines. The unique interaction mode with DNA and cancer cells inhibition effect clearly revealed the relationship between the structure and the activity of the novel antitumor agent Zn(II) complexes.
1,3-Dipolar cycloaddition reactions were studied to synthesize Polyhedral oligomeric silsesquioxane (POSS)-based norbornyl imide derivatives containing izoxazoline groups in good yields. And also 1,3-dipolar cycloaddition reactions of azomethine ylides with POSS-based norbornene dipolarophiles for a synthesis of the novel POSS-based norbornane-fused spiro-1,3-indandionolylpyrrolidines are reported. All newly synthesized POSS compounds were structurally characterized by FTIR, 1H, 13C NMR, HRMS and GC/MS analyses.
Directing the outcome of electrophilic addition reactions of norbornenes could be a challenging task. We have found that the ionic addition of bromine to dichloro-norbornene 2 is accompanied with the formation of a bromonium cation followed by anchimeric assistance of juxtaposed chlorine to give five-membered chloronium cation, which upon reacting with bromide gives norbornane 4 in >90% yield. At higher temperatures, however, the radical addition of Br2dominates so that dibromo compound 3 appears as the principal reaction product (72%). Stereo- and regioselective rearrangements of norbornenes, reported herein, could be of interest for the syntheses of complex haloalkanes.
A sequence of well controlled tert -butyldiphenylsilyl substituted poly(norbornene-dicarboximide)s with ascending molecular weights have been prepared using the Grubbs 1st generation catalyst in anhydrous chloroform. The kinetics of the polymerization was examined using nuclear magnetic resonance spectroscopy and gel permeation chromatography. By decreasing the Grubbs catalyst concentration, the polymer molecular weights increased linearly. The glass transition temperatures and thermal decomposition temperatures initially increased with polymer molecular weight, then reached a plateau. Regardless of molecular weight the polydispersities remained narrow. The polymers exhibited a predominantly trans microstructure and matrix-assisted laser desorption/ionization mass spectrometry was utilized to determine polymer end groups and to calculate the absolute molecular weights. The residual ruthenium content of the polymers was quantified using inductively coupled plasma mass spectrometry and the influence on the resistivity, refractive indices and thin film optical transmittance was evaluated.
We found that molecular baskets 1-3, with amino acids at their rim, undergo photoinduced decarboxylations to give baskets 4-6 forming a solid precipitate in water. Furthermore, organophosphonates 7-9 (OP), akin in size and shape to G-type nerve agents, form inclusion complexes with baskets 1-3 (K = 6-2243 M-1). Light irradiation (300 nm) of an aqueous solution of 1-3?OP led to the formation of precipitate containing an OP compound thereby amounting to a novel strategy for light-induced sequestration of nerve agents or, perhaps, other targeted compounds. Importantly, the stability of basket OP complexes in addition to functional groups at the basket's rim play a role in the efficiency (up to 98%) by which OPs are removed from water.
Two norbornene derivatives bearing a pendant pyridyl group were prepared: the 3-(pyridin-2-yl)propyl ester of 5-norbornene-endo-2-carboxylic acid (1) and N-(pyridin-2-ylmethyl)-5-norbornene-endo-2,3-dicarboxyimide (2). Both of these compounds produced high yields of ROMP polymers, poly(1) and poly(2), with the 2nd generation Grubbs catalyst. When Ag+ ions were added to these polymer solutions, the polymer-Ag+ composites, poly(1-Ag) and poly(2-Ag), were formed quantitatively. Poly(2) and poly(2-Ag) produced films from their DMF solutions, and the latter film showed strong antimicrobial properties against Gram-positive Bacillus subtilis and Gram-negative Escherichia coli. Alternatively, when the film of poly(2) was immersed in a solution of Ag+, it was able to trap the ion to give a surface-modified film [Ag/poly(2)]. The antimicrobial efficacy of [Ag/poly(2)] was the same as that of films made of poly(2-Ag), which indicated that the solid-state reaction of the film surfaces toward Ag+ ions in solution was quantitative. When the [Ag/poly(2)] film lost its biocidal effect after repeated use, the Ag + ions could be reloaded by immersing the film in a silver ion solution, which fully restored original activity.
Carbonic anhydrase (CA, EC 4.2.1.1) is a member of the metalloenzyme family. It catalyzes the rapid conversion of carbon dioxide (CO2) and water to bicarbonate (HCO3 ?) and protons (H+) and also plays an important role in biochemical and physiological processes. In this study, a number of novel 2-(4-(aryl)thiazole-2-yl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives were synthesized and evaluated for their inhibitory characteristics against the human CA isoenzymes I and II (hCA I and hCA II). The structures of the new molecules 8a–i were confirmed by means of IR, 1H NMR, 13C NMR, and elemental analysis. These compounds exhibited excellent inhibitory effects, in the low nanomolar range, with Ki values in the range of 27.07–37.80 nM against hCA I and in the range of 11.80–25.81 nM against hCA II. Our findings suggest that the new isoindolylthiazole derivatives have superior inhibitory effect over acetazolamide (AZA), which is used as clinical CA inhibitor with Ki values of 34.50 and 28.93 nM against the hCA I and hCA II isoenzymes, respectively.
The synthesis of (N-3,5-bis(trifluoromethyl)phenyl-exo-endo-norbornene-5,6-dicarboximide) as well as the ring opening methatesis polymerization (ROMP) of this monomer to yield poly(N-3,5-bis-(trifluoromethyl)phenyl-exo-encdo-norbornene-5,6-dicarboximide) are reported. The glass transition of the polymer is 162°C. Solubility coefficients of different gases (nitrogen, oxygen, carbon monoxide, carbon dioxide, methane, ethane, ethylene, propane, and propylene) in membranes prepared by casting from poly(N-3,5-bis(trifluoromethyl)phenyl-exo-endo-norbornene-5,6-dicarboximide) solutions were measured at several temperatures and pressures. The interpretation of the sorption results by the dual-mode model gives the Henry and Langmuir contributions to the solubility. As usual in glassy membranes, sorption processes are exothermic and the activation energies associated with the Henry and Langmuir parameters are also negative. Gas sorption in the continuous amorphous phase was interpreted in terms of the Flory-Huggins theory obtaining reasonable values for the enthalpic parameter x that accounts for the gas (in liquid form)-polymer interactions. The use of this approach to simulate gas sorption in polymers is discussed.
The regioselective synthesis of Polyhedral oligomeric silsesquioxane (POSS)-based norbornyl imide derivatives through palladium catalyzed Heck coupling reaction was reported on an effective synthetic method to organic–inorganic bio-hybrids serving as advanced materials. The reaction of POSS-based imide derivatives with various aryl iodides catalyzed by palladium acetate in the presence of triethyl amine, as the base, in DMF afforded the products in moderate yields. All new POSS derivatives were structurally characterized by FTIR, 1H, 13C NMR, HRMS and GC/MS analyses.
Two new novel complexes, [Cu4(Endc)4(phen)4]?7(H2O)?2(O) and [Mn2(Endc)2(phen)2(H2O)2]?(H2O) (phen =1,10-phenanthroline, H2Endc?=?endo-norbornene-cis-5,6-dicarboxylic acid), were synthesized and structurally characterized using IR and 1H NMR spectroscopies, elemental analysis and single-crystal X-ray diffractometry. Their reactivity with calf thymus DNA and HeLa cell DNA was measured using UV absorption and fluorescence spectroscopies. The results indicated that these complexes can bind to DNA with different binding affinity. Gel electrophoresis assay demonstrated the ability of the complexes to cleave pBR322 plasmid DNA. Apoptotic study showed that the complexes exhibit significant cancer cell inhibitory rates. Eventually, the complexes can suitably dock with a special DNA (PDB ID: 1AIO).
The reaction of benzothiete (1) and the bicyclic alkenes 5, 7, 9, or 12 shows a very high π side selectivity (de > 95%) in the formation of the polycyclic thiopyrans 6, 8, 10, 11, 13, and 14.
-
The structurea€"activity requirement of the PP2A-inhibiting demethylcantharidin for the circumvention of cisplatin cross-resistance demonstrated by the novel TCMa€"Pt compounds is discussed. Novel TCM-platinum compounds [Pt(C8H8O5)(NH 2R)2] 1-5, derived from integrating demethylcantharidin, a modified component from a traditional Chinese medicine (TCM) with a platinum moiety, possess anticancer and protein phosphatase 2A inhibition properties. The compounds are able to circumvent cisplatin resistance by apparently targeting the DNA repair mechanism. Novel isosteric analogues [Pt(C9H 10O4)(NH2R)2] A and B, devoid of PP2A-inhibitory activity, were found to suffer from an enhanced DNA repair and were cross-resistant to cisplatin. The results advocate a well-defined structure-activity requirement associating the PP2A-inhibiting demethylcantharidin with the circumvention of cisplatin cross-resistance demonstrated by TCM-Pt compounds 1-5.
Remarkably strong binding of the new [5]polynorbornane based host 2b to the terephthalate dianion is based on size complementarity of the preorganised binding cleft with the rigid dicarboxylate guest. The Royal Society of Chemistry.
We have determined the crystal structures of bicyclo[2.2.1]hept-2-ene-endo-cis-5,6-dicarboxylic anhydride, C9H8O3, (I), 1,2,3,4,7,7-hexachlorobicyclo [2.2.1]hept-2-ene-endo-cis-5,6-dicarboxylic anhydride diethyl ether solvate, C9H2Cl6O3·0.16-C4H10O, (II), bicyclo[2.2.1]hept-2-ene-endo-cis-5,6-dicarboxylic acid, C9H10O4, (III), 1,2,3,4,7,7-hexachlorobicyclo [2.2.1]hept-2-ene-endo-cis-5,6-dicarboxylic acid, C9H4Cl6O4, (IVa) and (IVb), and ethyl 1,2,3,4,7, 7-hexachloro-6-carboxybicyclo[2.2.1]hept-2-ene-endo-cis-5-carboxylate monohydrate, C11H8Cl6O4·H2O, (V). Compounds (I) and (II) were prepared by a standard Diels-Alder reaction from maleic anhydride and cyclopentadiene or hexachlorocyclopentadiene, respectively. The crystal-growing processes of these compounds led to surprising results: rapid recrystallization of (I) from diethyl ether and (II) from petroleum ether gave crystals of these compounds, however, crystallization by slow evaporation techniques using common solvents yielded new compounds in which the five-membered heterocycle has been cleaved.
Selectivity is demonstrated in a supramolecular host:guest system using a receptor with a non-linear binding site. For the "open" receptor 1 strong binding for both flexible and rigid guests was observed. Receptor 2, with a "blocked" binding site, also bound flexible guests effectively but its affinity for rigid guests was 50 fold lower.
Palladium-catalyzed, regioselective hydroarylation reactions of N-norbornenylimide substituted amides were studied to synthesize pentanamide derivatives containing exo-aryl-substituted norbornyl imide groups in excellent yields. All newly synthesized derivatives have been characterized by FTIR, 1H, 13C NMR, GC/MS and TOF/Qtof analyses.
-
A series of bis-thiourea-functionalized [n]polynorbornane hosts (1-6) with increasing size were synthesized and their anion-binding properties were evaluated by using 1HNMR spectroscopic titration and Job's plot analysis. The larger bis-thiourea-[3]polynorbornane scaffolds 4 and 5 bound acetate in a 1:1 (cooperative) arrangement, whereas the corresponding smaller norbornane host 2, identical in preorganization, bound acetate in a 1:2 (independent) arrangement. In contrast, the size of the framework had no influence on the binding of dihydrogenphosphate. These results clearly highlight the subtle influence that the framework itself can have on host-guest interactions. One anion or two? A series of bis-thiourea-functionalized [n]polynorbornane hosts (1-6) with increasing size were synthesized and their anion-binding properties were evaluated by using 1HNMR spectroscopic titration and Job's plot analysis. Our results highlight the subtle influence that the framework itself can have on host-guest interactions.
Comparative studies were carded out on boron trifluoride etherate, phenylboron difluoride and meta-nitrophenylboron difluoride for the Lewis acid catalysed Diels-Alder reaction of cyclopentadiene and a range of standard dienophiles in tetrahydrofuran solution. Phenylboron difluoride showed remarkably similar reactivity in terms of yield and endo to exo selectivity to boron trifluoride, whereas meta-nitrophenylboron difluoride was more reactive than either boron trifluoride or phenylboron difluoride and showed more marked differences in endo: exo ratios. These results contrast to some extent with gas-phase semi-empirical calculations (PM3), which suggest that boron trifluoride and meta-nitrophenylboron difluoride should have similar reactivity; phenylboron difluoride being less reactive. However, since arylboron difluorides are easily prepared, these Lewis acids represent a group of potentially highly tuneable catalysts for Diels-Alder reactions.
A new oxa-cage natural product daphniacetal A (1) was isolated from Daphniphyllum macropodum Miq. Its structure and relative configuration were established based on spectroscopic data and the single-crystal X-ray diffraction crystallography. Compound 1 was also synthesized for determination of its absolute configuration and evaluation of antioxidant effects.
The palladium-catalysed hydroarylation of unsaturated N-acylamino- substituted tricyclic imides provided a new stereoselective synthesis of exo-aryl(heteroaryl)-substituted tricyclic N-acylamino imides.
Protein kinase C (PKC) is a widely studied molecular target for the treatment of cancer and other diseases. We have approached the issue of modifying PKC function by targeting the C1 domain in the regulatory region of the enzyme. By using the X-ray crystal structure of the PKCδ C1b domain combined with molecular modeling, we discovered (3-aminodecahydro-1,4- methanonaphthalen-2-yl)methanol as a novel C1 domain ligand. The stereoselective synthesis of this tricyclic γ-amino alcohol was based on two successive Diels-Alder reactions to construct the six continuous stereocenters of the key intermediate.
Synthesis of a number of novel, conformationally rigid β-amino esters has been achieved via a tandem olefin metathesis reaction. The starting materials are readily accessible from the Diels-Alder adduct between cyclopentadiene and maleic anhydride. Georg Thieme Verlag Stuttgart.
One unsaturated polymer support was prepared through ring opening metathesis polymerization (ROMP) of norbornene-2,3-dip-toluene sulfonate initiated by Grubbs 2nd initiator and manganese porphyrins were immobilized on polymer through transesterification reaction. To investigate the effect of C[dbnd]C bonds along polymer chains on the catalytic behavior, the obtained polymer supported catalyst (P-PPIXMnCl) was applied in oxidation of low concentration Fe2+ to mimic catalytic behavior of Ceruloplasmin. In the presence of P-PPIXMnCl, the conversion of Fe2+ reaches to 91.92% and 96.46% at 10 °C and 37.5 °C (body temperature), respectively. Compared to manganese porphyrins, P-PPIXMnCl can dramatically increase oxidation rate of Fe2+ and the catalytic kinetic shows that the oxidation reaction changes from second-order to third-order. Upon hydrogenation of ROMP polymer, the oxidation reaction still conforms to the second-order kinetics. Density functional theory (DFT) calculation shows that the C[dbnd]C bonds along polymer chains play an important role in the coordination with Fe2+ in the catalytic microenvironment. The real time morphology of supported catalysts in aqueous environment characterized by Cryo-TEM indicates that hydrogenation can shrink the morphology of polymer-water skeleton. The catalyst could be recycled six times without any significant loss in activity. The liner heterogeneous catalyst is expected to be used as drugs for treating excessive iron accumulation in the human body.
(Chemical Equation Presented) A new family of [3]polynorbornane frameworks exhibiting conformationally preorganized aromatic thiourea (cleft-like) receptors have been designed and synthesized for anion recognition. These show excellent affinity for the biologically relevant dihydrogenphosphate (H 2PO4-) and dihydrogenpyrophosphate (H 2P2O72-) anions (among others), which are bound in 1:1 and 2:1 (host:anion) ratio, respectively. Moreover, visually striking color changes accompany guest binding, enabling this family to act as colorimetric anion sensors.
A novel nine-step diastereoselective route to the ABCD ring system of the natural product rubriflordilactone B is reported. Use of an α-substituted butenolide derived from maleic anhydride facilitated a 1,4-conjugate addition to provide a diene. The order in which a ringclosing metathesis and enolate oxidation were performed on this compound dictated the relative stereochemistry of the target. The final product exhibited anisotropic effects during roomerature NMR studies, requiring elevatederature experiments to confirm its identity.
In the field of our research interest, we designed new sulfa drugs–substituted norbornyl imides as prospective bioactive scaffolds by the reaction of endo-endic anhydride with sulfa drugs followed by reductive Heck reactions of these products. Norbornenyl imides, as starting compounds, and their Heck products were achieved in good yields. Molecular characterization and stereochemistry of the compounds were investigated using Fourier transform infrared, liquid chromatography time-of-flight mass spectrometry, proton nuclear magnetic resonance, carbon 13 attached proton test, H-H correlation spevtroscopy, and nuclear overhauser enhancement spectroscopy experiments. The Heck products of norbornenyl imides showed exo-selectivity. All eight novel synthesized compounds, including two sulfa drugs–based norbornenyl imides and six exo-5-aryl- substituted norbornyl imides, as well as standard sulfonamides, were evaluated for antimicrobial activity against nine microorganisms consisting of Gram-negative bacteria, Gram-positive nonactinobacteria, actinobacteria, and yeast. The most active compound was 3a against actinobacteria. The efficacy of 2 and its derivatives against the studied strains were weaker than 3 and its derivatives, but all the studied samples exhibited antifungal potency.
The preparation method comprises the following steps: (1) adding a solvent in a reaction container, maleic anhydride, a chiral catalyst D and nitrogen to replace air in a reaction container. (2) At -5 - 5 °C, cyclopentadiene is added dropwise, and the dropping is carried out at a certain temperature. (3) After the completion of the reaction, water was added and quenched. Concentration, crystallization in ethanol and filtration gave exo. The preparation method has the characteristics of high reaction stereoselectivity, high yield, good product quality, simple and convenient process operation, high stability and high safety, and is suitable for industrial mass production.
Neurodegenerative disorders are characterised by progressive loss of neuronal functions. Of the proposed mechanisms, excitotoxicity, mediated by prolonged glutamate activation and calcium overload, is prominent. NGP1-01, a polycyclic cage amine, and tricyclo[6.2.1.02,7]undec-9-ene-3,6-dione have been shown to display calcium-modulating properties. In this study, we synthesised structurally-related 4-oxatricyclo[5.2.1.02,6]dec-8-ene-3,5-dione as the base scaffold, and incorporated various functional moieties through aminolysis, to afford a series of imide derivatives. All final compounds were obtained in yields between 47-97% and their structures were confirmed by NMR, IR and MS. These structurally-related derivatives could potentially act as neuroprotective agents. Additionally, their synthetic versatilities could make them precursors, as lead compounds, to potential pharmacologically-active agents.
![(1R,2S,3R,4S)-3-(2,2-Difluoro-ethylcarbamoyl)-bicyclo[2.2.1]hept-5-ene-2-carboxylic acid](/upload/2023/2/595c026f-dd3a-485a-8cfc-70194b988ba0.png)
(1R,2S,3R,4S)-3-(2,2-Difluoro-ethylcarbamoyl)-bicyclo[2.2.1]hept-5-ene-2-carboxylic acid

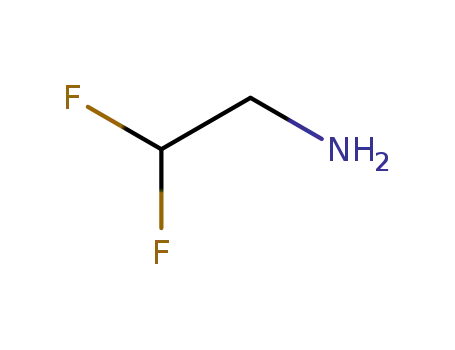
2,2-difluorethylamine

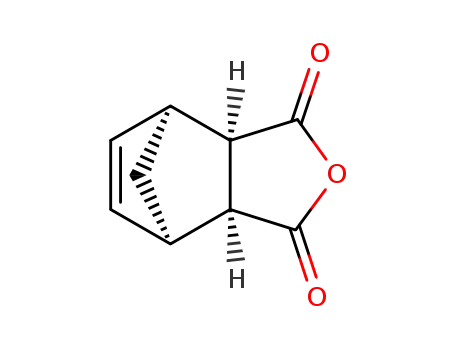
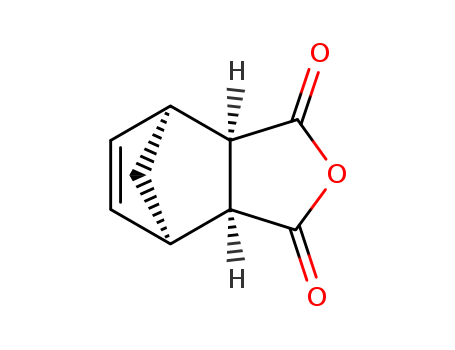
3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride
| Conditions | Yield |
|---|---|
|
With
water;
at 38 ℃;
Rate constant;
|
(1R,7S)-5-(2,2-Difluoro-ethylamino)-5-hydroxy-4-oxa-tricyclo[5.2.1.02,6]dec-8-en-3-one


2,2-difluorethylamine


3,6-endomethylene-1,2,3,6-tetrahydrophthalic anhydride
| Conditions | Yield |
|---|---|
|
With
water;
at 38 ℃;
Rate constant;
|
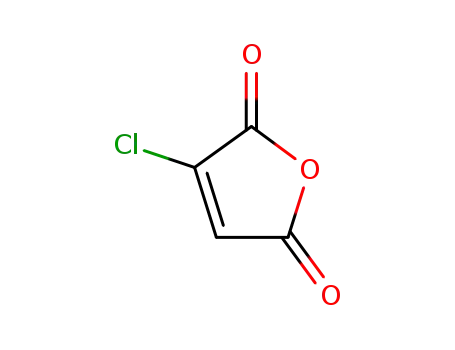
2-chloromaleic anhydride

endo-Dicyclopentadien
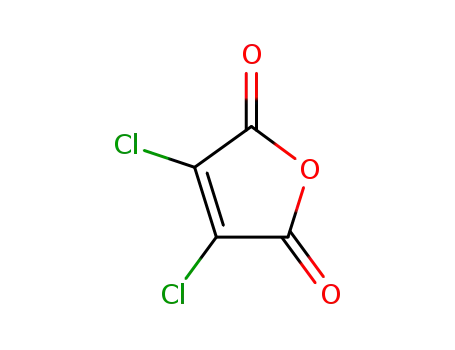
dichloromaleic acid anhydride
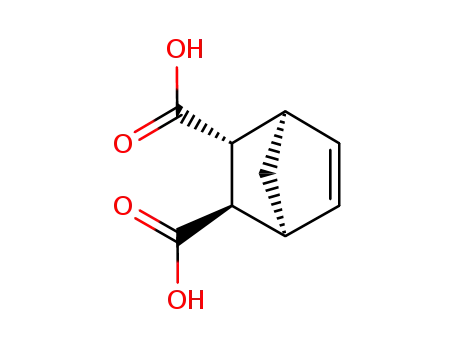
norbornene-5,6-dicarboxylic acid
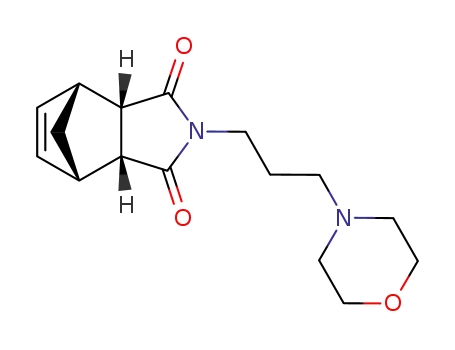
2-(3-morpholino-propyl)-(3ac,7ac)-3a,4,7,7a-tetrahydro-4r,7c-methano-isoindole-1,3-dione
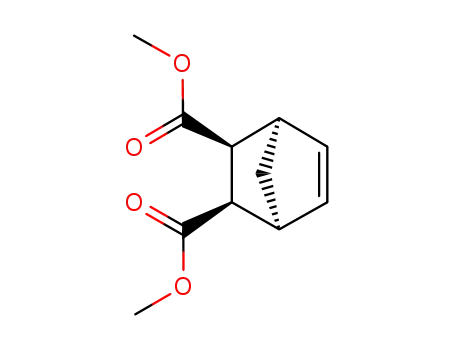
endo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic dimethyl ester
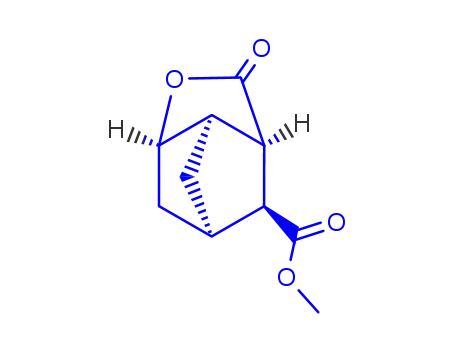
endo-3-methoxycarbonyl-2,6-norbornanecarbolactone
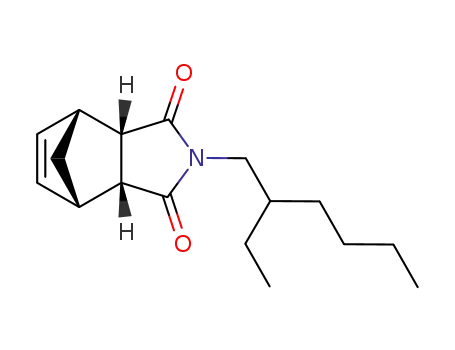
(+/-)-2-(2-ethyl-hexyl)-(3ac,7ac)-3a,4,7,7a-tetrahydro-4r,7c-methano-isoindole-1,3-dione
CAS:161265-03-8
Molecular Formula:C39H32OP2
Molecular Weight:578.6
CAS:16839-97-7
CAS:25550-51-0
CAS:25134-21-8