Your Location:Home >Products >OLED intermediates >Fluorenes >393841-81-1

Product Details
|
General Description |
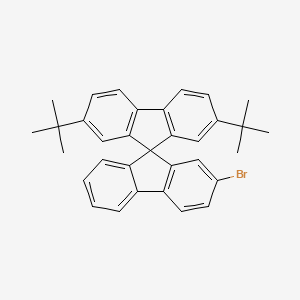
2'-BroMo-2,7-di-tert-butyl-9,9'-spirobi[fluorene] is a chemical compound consisting of a spirobi[fluorene] core with two tert-butyl groups attached to the 2 and 7 positions and a bromine atom at the 2' position. 2'-broMo-2,7-di-tert-butyl-9,9'-spirobi[fluorene] is often used as a building block in organic synthesis and materials science due to its unique spirobi[fluorene] structure. It has been studied for its potential use in organic light-emitting diodes (OLEDs) and other optoelectronic devices. The tert-butyl groups provide steric hindrance, making the molecule bulky and hindering intermolecular interactions, which can be advantageous for certain applications. The bromine atom can also be utilized for further functionalization of the molecule. 2'-Bromo-2,7-di-tert-butyl-9,9'-spirobi[fluorene] is available for purchase from Puyang Huicheng Electronic Material Co., Ltd. |
| Uses | 2-Bromo-2,7-di-tert-butyl-9,9'-spirobi[fluorene] is a useful building block in the synthesis of novel functional materials. The spiro-fused fluorene core provides rigidity and thermal stability, while the bromine atom enables further derivatization through cross-coupling reactions. The tert-butyl substituents enhance the solubility and processability of the compound, facilitating its incorporation into various optoelectronic devices, such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), and organic photovoltaics (OPVs). Overall, 2-Bromo-2,7-di-tert-butyl-9,9'-spirobi[fluorene] is a versatile and important compound in the field of organic electronics and materials science. |
The invention discloses an organic elect...
A series of novel biscyclometallated iri...
The invention provides phosphorescent or...
The sterically hindered and highly therm...
2-bromofluoren-9-one

2-bromo-(2',7'-di-tert-butyl)-9,9'-spirobifluorene
| Conditions | Yield |
|---|---|
|
2-bromo-4,4'-di-tert-butylbiphenyl; With n-butyllithium; In tetrahydrofuran; hexane; at -78 ℃; for 1h;
2-bromofluoren-9-one; In tetrahydrofuran; hexane; at -78 ℃;
With hydrogenchloride; acetic acid; for 1h; Heating / reflux;
|
54% |
|
Multi-step reaction with 2 steps
1.1: Mg; BrCH2CH2Br / diethyl ether / 8 h / Heating
1.2: diethyl ether / Heating
2.1: HCl; acetic acid / H2O / 1 h / Heating
With hydrogenchloride; magnesium; acetic acid; ethylene dibromide; In diethyl ether; water;
|
2,7-di-tert-butyl-9,9'-spirobi[9H-fluorene]

2-bromo-(2',7'-di-tert-butyl)-9,9'-spirobifluorene
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; sulfuric acid; In dichloromethane; at 20 ℃; for 12h;
|
33.8% |
2-bromo-9-(4,4'-di-tert-butyl-biphenyl-2-yl)-9H-fluoren-9-ol
2-bromofluoren-9-one
2-bromo-4,4'-di-tert-butylbiphenyl
2,7-di-tert-butyl-9,9'-spirobi[9H-fluorene]
[(2',7'-di-tert-butyl)-9,9'-spirobifluoren-2-yl]phenylamine
CAS:28320-31-2
Molecular Formula:C<sub>15</sub>H<sub>13</sub>Br
Molecular Weight:273.17
CAS:400607-31-0
CAS:1198395-24-2
Molecular Formula:C27H23N
Molecular Weight:361.5