Your Location:Home >Products >OLED intermediates >Thiophenes >1693-86-3

Product Details
Description
3-Hexylthiophene is the intermediate for the synthesis of poly(3-hexylthiophene), referred as P3HT. To date, it is the most studied polymer for polymer solar cells. The efficiency of a P3HT/PCBM solar cell is typically 4-5 %, but with new fullerene materials developed to closely match the energy levels of P3HT (HOMO 5.0 eV, LUMO 3.0 eV), device performances have been pushed to 6.5%.
Chemical Properties
colorless transparent or light yellow liquid
Uses
3-n-Hexylthiophene is a conducting polymer precursor. may be used in the synthesis of poly(3-hexylthiophene). MgKR X-ray and low energy electron induced oligomerization of physisorbed layers of 3-hexylthiophene condensed on clean gold results in the formation of an organic film.
Uses
3-Hexylthiophene may be used as starting reagent in the synthesis of poly(3-hexylthiophene) (P3HT). Poly(3-hexylthiophene) (P3HT) nanofibres have been used for the preparation of organic phototransistors (OPTs).
Preparation
The 2,5-dibromo-3-dodecylthiophene was dissolved in tetrahydromyran, added with methylmagnesium bromide, preheated and added with catalyst for reaction reaction was injected into alcohol solvent, and hexane was added to remove the copolymer, and then the mixture was soxhlet filtered using chloroform, and the chloroform layer was evaporated and concentrated to obtain a purple membrane; the purple membrane was vacuum filtered to obtain the product 3-hexylthiophene.
General Description
3-Hexylthiophene, a sulfur containing heterocyclic building block, is a thiophene derivative. Poly(3-hexylthiophene) (P3HT) nanofibres have been used for the preparation of organic phototransistors (OPTs).
InChI:InChI=1/C10H16S/c1-2-3-4-5-6-10-7-8-11-9-10/h7-9H,2-6H2,1H3
Three novel low-bandgap copolymers containing alkylated 4,7-dithien-2-yl-2,1,3-benzothiadiazole (HBT) and different electron-rich functional groups (dialkylfluorene (PFV-HBT), dialkyloxyphenylene (PPV-HBT) and dialkylthiophene (PTV-HBT)) were prepared by Horner polycondensation reactions and characterized by 1H NMR, gel permeation chromatography, and elemental analysis. The alkyl side chain brings these polymeric materials good solubility in common organic solvents, which is critical for the manufacture of solar cells in a cost-effective manner. The copolymers exhibit low optical bandgap from 1.48 to 1.83 eV. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of the copolymers were measured by cyclic voltammetry. Theoretical calculations revealed that the variation laws of HOMO and the LUMO energy levels are well consistent with cyclic voltammetry measurement. The bulk heterojunction photovoltaic devices with the structure of ITO/PEDOT-PSS/polymer:PCBM/LiF/Al were fabricated by using the three copolymers as the donor and (6,6)-phenyl-C61-butyric acid methyl ester (PCBM) as the acceptor in the active layer. The device based on PTV-HBT:PCBM (1:4 w/w) achieved a power conversion efficiency of 1.05% under the illumination of AM 1.5, 100 mW/cm2.
Polymer nanocomposites (PNCs) of poly(3-hexylthiophene) (P3HT) with organically modified montmorillonite (om-MMT) clay have been prepared by the solvent casting method. WAXS and TEM studies indicate exfoliated clay structure for lower clay content, but at higher clay content (5%, w/w) intercalated structures appear. The interchain lamella of P3HT exists in the nanocomposite, and the P3HT crystals become more ordered, showing better X-ray diffraction peaks. The thermal stability of PNCs increases significantly, and 1% clay content PNC exhibits the maximum thermal stability. The glass transition temperature (Tg), β-transition temperature (Tβ), the melting point (Tm), and the enthalpy of fusion (ΔT) of the PNCs are increased as compared to those of pure P3HT. The storage modulus (G′) of PNCs showed a dramatic increase from that of pure P3HT, and the increase is larger in the temperature range 20-50 °C. The FTIR study indicates a decrease in Si-O-Si and Si-O stretching frequency for the exfoliated clay structure. The UV-vis study showed a blue shift of the π-π*transition band of P3HT in the PNCs, and they exhibit photoluminescence quenching which increases with increase in clay concentration. The dc conductivity of undoped PNCs remains almost the same as that of pure P3HT, but iodine-doped PNCs, however, exhibit 2.5-3 times greater conductivity than that of iodine-doped P3HT.
The reaction of bromide or iodide with ate-complexes obtained from trialkylboranes and 3-lithiofuran or 3-lithiothiophene gives the corresponding 3-alkylfurans or 3-alkylthiophenes in good yields, respectively.
Thiophene-based rings are one of the most widely used building blocks for the synthesis of sulfur-containing molecules. Inspired by the redox diversity of these features in nature, we demonstrate herein a redox-divergent construction of dihydrothiophenes, thiophenes, and bromothiophenes from the respective readily available allylic alcohols, dimethyl sulfoxide (DMSO), and HBr. The redox-divergent selectivity could be manipulated mainly by controlling the dosage of DMSO and HBr. Mechanistic studies suggest that DMSO simultaneously acts as an oxidant and a sulfur donor. The synthetic potentials of the products as platform molecules were also demonstrated by various derivatizations, including the preparation of bioactive and functional molecules.
A series of highly emissive π-conjugated A-alt-B type copolymers (P1-P3) appended with a 1,2,3-triazole moiety was synthesized via Suzuki polymerization. The well-defined and soluble π-conjugated copolymers were characterized via multinuclear NMR spectroscopy and tetradetector GPC studies, showing a molecular weight (Mn) in the range of 16.4-20.1 kDa with a polydispersity index in the range of 1.25-1.42. The synthesized emissive π-conjugated polymer probes were explored as fluorescent chemosensors for nitroaromatic compounds (NACs) in solution, vapor and contact mode. Detailed photophysical and sensing studies were performed to understand the polymer-NAC interaction, inducing the selective fluorescence quenching of the π-conjugated polymer probes through the photoinduced electron transfer (PET) mechanism. All the polymeric probes (P1-P3) were highly reversible in nature with NACs, and thus could be reused multiple times. The limit of detection of the probes towards nitroaromatics was found to be in the range of 120-200 ppb with a high association constant in the order of 104 M-1. Furthermore, test paper kits were also fabricated, which allowed the trace detection of picric acid by the naked eye, making it a practical means for the quick, easy and inexpensive on-site detection of NAC-based explosives.
Organolithium compounds are amongst the most important organometallic reagents and frequently used in difficult metallation reactions. However, their direct use in the formation of C?C bonds is less established. Although remarkable advances in the coupling of aryllithium compounds have been achieved, Csp2?Csp3 coupling reactions are very limited. Herein, we report the first general protocol for the coupling or aryl chlorides with alkyllithium reagents. Palladium catalysts based on ylide-substituted phosphines (YPhos) were found to be excellently suited for this transformation giving high selectivities at room temperature with a variety of aryl chlorides without the need for an additional transmetallation reagent. This is demonstrated in gram-scale synthesis including building blocks for materials chemistry and pharmaceutical industry. Furthermore, the direct coupling of aryllithiums as well as Grignard reagents with aryl chlorides was also easily accomplished at room temperature.
Synthesis of two conducting polymers containing 3-hexylthiophene and 3-[2-(2-(2-methoxyethoxy)ethoxy)ethoxy]thiophene is demonstrated. In thin-film transistors, the high-molecular-weight polymer shows an average mobility of 4.2 × 10?4 cm2 V?1 s?1. Most importantly, the polymers have high conductivity upon doping with iodine and also have high stability in the doped state with high conductivities measured even after 1 month. Furthermore, the doping causes transparency to thin films of the polymer and the films are resistant to common organic solvents. All these properties indicate a great potential for the iodine-doped polymer to be used as an alternative to commercially available poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate).
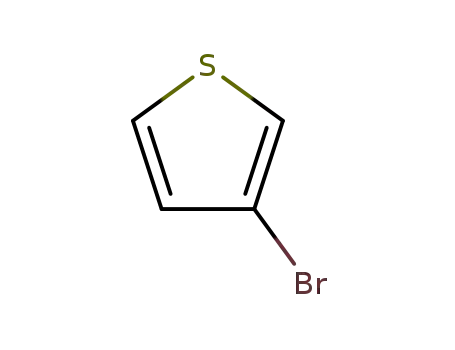
3-Bromothiophene

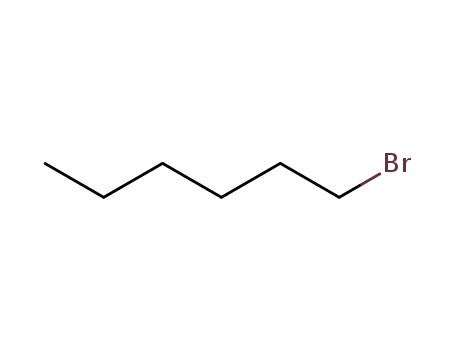
1-bromo-hexane

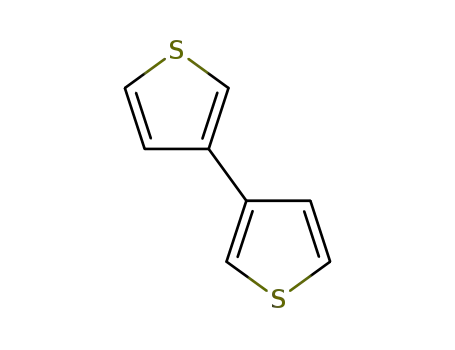
3,3'-bithiophene


3-(1-methylpentyl)thiophene


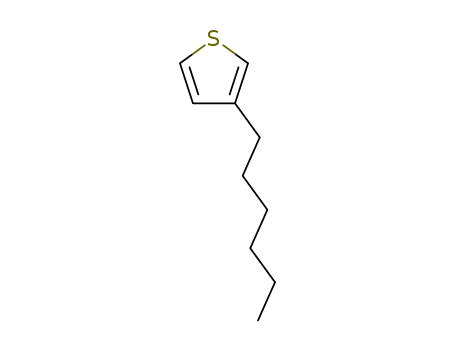
3-hexylthiophene
| Conditions | Yield |
|---|---|
|
1-bromo-hexane; n-hexylmagnesium bromide;
With
magnesium;
In
2-methyltetrahydrofuran;
at 60 - 85 ℃;
for 4.5h;
3-Bromothiophene;
1,3-bis[(diphenylphosphino)propane]dichloronickel(II);
In
2-methyltetrahydrofuran;
at 15 - 20 ℃;
for 20h;
With
hydrogenchloride; water;
In
2-methyltetrahydrofuran;
Product distribution / selectivity;
|
97.5 - 98.88 %Chromat. 0.37 - 0.45 %Chromat. 0.15 - 0.49 %Chromat. |

3-Bromothiophene

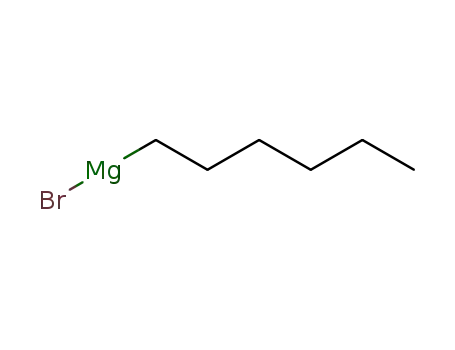
n-hexylmagnesium bromide


3,3'-bithiophene


3-hexylthiophene
| Conditions | Yield |
|---|---|
|
bis(triphenylphosphine)nickel(II) chloride;
In
tetrahydrofuran;
at 0 - 60 ℃;
for 2.5h;
Product distribution / selectivity;
|
36.2 - 82.7 %Chromat. 6.4 - 27.1 %Chromat. |
|
bis-triphenylphosphine-palladium(II) chloride;
In
diethyl ether;
at 20 ℃;
for 2.5h;
Product distribution / selectivity;
|
4.0 - 4.5 %Chromat. 0.2 - 0.5 %Chromat. |
|
bis(triphenylphosphine)nickel(II) chloride;
In
diethyl ether;
at 20 ℃;
for 1h;
Product distribution / selectivity;
|
1.7 - 3.1 %Chromat. 0.5 - 6.3 %Chromat. |
|
In
diethyl ether;
at 20 ℃;
for 1h;
Conversion of starting material;
|
0.5 - 1.1 %Chromat. 0.2 - 0.4 %Chromat. |

3-Bromothiophene
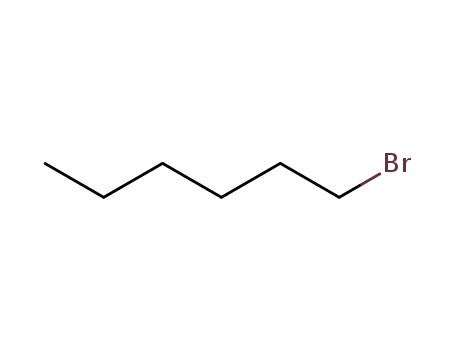
1-bromo-hexane

Trihexylboran; Tri-n-hexylbor
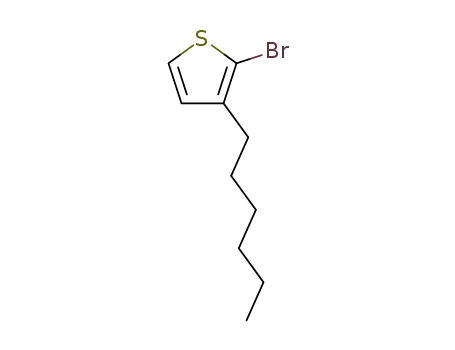
3-hexyl-2-bromothiophene

3-hexyl-2-bromothiophene
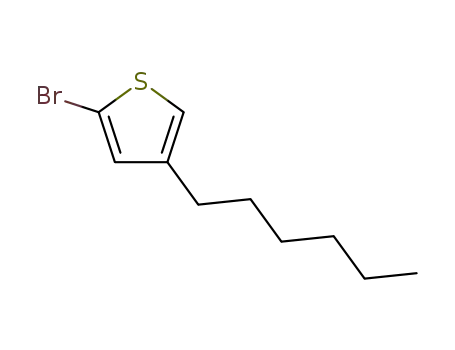
2-bromo-4-n-hexylthiophene
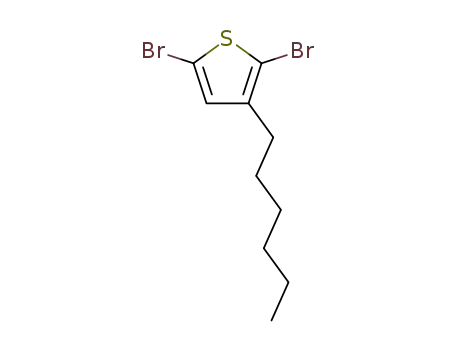
2,5-dibromo-3-hexylthiophene
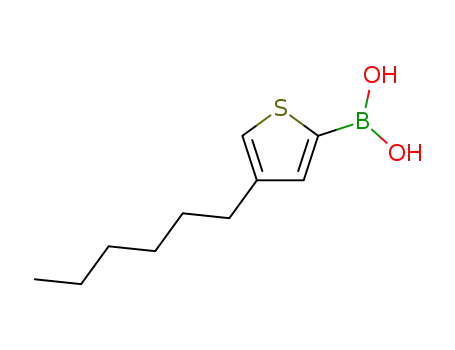
(4-hexylthiophen-2-yl)boronic acid
CAS:84680-95-5
CAS:18794-77-9
CAS:1081-34-1