Your Location:Home >Products >Organic phosphines >Phenyl phosphines >15133-82-1

Product Details
Chemical Properties
rust-brown to brown fine powder
Uses
Tetrakis(triphenylphosphine)nickel(0) is a catalyst for coupling reactions of organic halides, organometallic compounds and also for the cyclooligomerization of cumulenes.
Uses
Catalyst for coupling reactions of organic halides, organometallic Compounds and also for the cyclooligomerization of cumulenes;.
InChI:InChI=1/4C18H15P.Ni/c4*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;/h4*1-15H;
The nickel-catalyzed [2 + 2] annulations of electron-deficient allenes proceed efficiently in a highly regioselective manner under very mild conditions to give the head-to-head cyclodimerization products, bismethylenecyclobutanes, as single isomers in good to fair yields. We also carried out the stoichiometric reaction of these allenes in the presence of Ni(0) complexes and elucidated the mechanism of this highly selective reaction.
Trisubstituted silanes, e.g., Me,.(EtO)(3-n)SiH (where n = 0-2) and Me2PhSiH in the presence of nickel complexes, e.g., [Ni(acac)2] and [Ni(cod)2], undergo two reactions of dehydrogenative silylation of styrene to yield in
Controlling the behavior of terminal alkynes in metal-catalyzed intermolecular tandem reactions is a formidable challenge despite the potential advantage offered by these strategies in modern synthesis. Herein, we describe that a nickel catalyst enables a tandem process involving the rapid dimerization of terminal alkynes into 1,3-enynes and the cycloaddition of these intermediates with an azetidinone, an oxetanone or benzocyclobutenones. Significantly, the slow or sequential addition of reagents and catalysts is not required to orchestrate their reactivity. These results are in stark contrast with previous cycloadditions of terminal alkynes with strained four-membered ring substrates, which previously led to oligomerization or cyclotrimerization, except in the case of tert-butylacetylene.
Studies into the mechanism of 8-aminoquinoline-directed nickel-catalyzed C(sp3)-H arylation with iodoarenes were carried out, to determine the catalyst resting state and optimize catalytic performance. Paramagnetic complexes undergo the key C-H activation step. The ubiquitous base Na2CO3is found to hinder catalysis; replacement of Na2CO3with NaOtBu gave improved catalytic turnovers under milder conditions. Deprotonation of the 8-aminoquinoline derivativeN-(quinolin-8-yl)pivalamide (1a) at the amide nitrogen using NaH, followed by reaction with NiCl2(PPh3)2allowed for the isolation of complex Ni([AQpiv]-κN,N)2(3) with chelating N-donors (where [AQpiv] = C9NH6NCOtBu). Complex3is a four-coordinate disphenoidal high-spin Ni(II) complex, excluding short anagostic Ni-tBu hydrogen interactions. Complex3reacts with the paddle-wheel [Ph3PNi(μ-CO2tBu)2]2(6·PPh3) ortBuCO2H to give insoluble {[AQpiv]Ni(O2CtBu)}2(5). Dissolution of5in donor solvents L (L= DMSO and DMF) gave a paramagnetic intermediate assigned by NMR as [AQpiv]Ni(O2CtBu)L (5·L) and equilibrium reformation of3and6·L. DFT calculations support this equilibrium in solution. Both3and5undergo C-H activation at temperatures as low as 80 °C and in the presence of PR3(PR3= PPh3, PiBu3) to give Ni(C9NH6NCOCMe2CH2-κN,N,C)PR3(7·PR3). The C-H functionalization reaction orders with respect to7·PiBu3, iodoarenes, and phosphines were determined. Hammett analysis using electronically different aryl iodides suggests a concerted oxidative addition mechanism for the C-H functionalization step; DFT calculations were also carried out to support this finding. When Na2CO3is used as the base, the rate determination step for C-H functionalization appears to be 8-aminoquinoline deprotonation and binding to Ni. The carbonate anion was also observed to provide a deleterious NMR-inactive low-energy off-cycle resting state in catalysis. Replacement of Na2CO3with NaOtBu improved catalysis at milder conditions and made carboxylic acid and phosphine additives unnecessary. Complex3and its functionalized analogues were observed as the catalyst resting state under these conditions.
The activation and reduction of nickel(II) salts under conditions relevant to Ni-catalyzed borylation reactions is reported. Methanolic solutions of NiCl2·6H2O reacted with >2 equiv of (iPr)2NEt were converted to polymeric Ni(OMe)2, which was characterized by IR spectroscopy, magnetic susceptibility measurements, and verified by independent synthesis from NaOMe. When diboron reagents such as bis(neopentylglycolato) diboron (B2(npg)2) were exposed to methanolic solutions of Ni(II) salts and (iPr)2NEt, nickel metal was deposited along with the evolution of hydrogen gas. A direct relationship between yield of nickel metal and equivalents of B2(npg)2 relative to [Ni] was also observed, reaching >99% yield at 8 equiv. Ni(0) coordination complexes were also isolated when a phosphine, phosphite, and/or diene ligand was present, all starting from NiCl2·6H2O, including the following: Ni[P(OPh)3]4 (74% yield), Ni[P(OiPr)3]4 (54% yield), Ni(PPh3)4 (75% yield), (dppp)2Ni + Ni(1,5-cod)2 (dppp = 1,3-bis(diphenylphosphine)propane) (91% yield), Ni(1,5-cod)2 (1,5-cod = 1,5-cyclooctadiene) (69% yield), and (dppf)Ni(1,5-cod) (dppf = 1,1′-bis(diphenylphosphino)ferrocene) (84% yield). The high yields observed indicated the efficient reduction of Ni(II) to Ni(0) and a likely route for precatalyst entry into the Ni-borylation catalytic cycle. These in situ reduction conditions were also successfully applied to a previously developed Ni-catalyzed alpha-arylation reaction where the requisite Ni(1,5-cod)2 precatalyst was substituted for NiCl2·6H2O and catalytic diboron. Comparable yields to the original report were observed under these conditions, further demonstrating that Ni(0) active species can be efficiently accessed with diboron reagents under protic conditions from Ni(II) salt hydrates.
The present invention relates to air stable, binary Ni(0)-olefin complexes and their use in organic synthesis.
chlorotris(triphenylphosphine)nickel

bis(triphenylphosphine)nickel(II) chloride

tetrakis(triphenylphosphine)nickel(0)
| Conditions | Yield |
|---|---|
|
With
tetrabutyl-ammonium chloride;
In
tetrahydrofuran;
at 23 ℃;
Inert atmosphere;
Glovebox;
|
triphenylphosphine

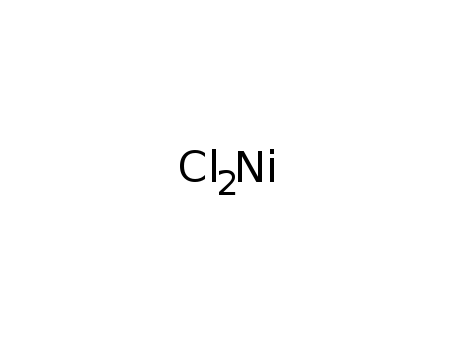
nickel dichloride

tetrakis(triphenylphosphine)nickel(0)
| Conditions | Yield |
|---|---|
|
With
zinc;
In
N,N-dimethyl-formamide;
at 55 ℃;
for 3h;
Inert atmosphere;
|
82% |
|
With
zinc;
In
N,N-dimethyl-formamide;
starting materials in dmf at 50°C with zinc dust as reducing agent;
|
|
|
With
zinc;
In
N,N-dimethyl-formamide;
at 55 - 60 ℃;
for 1.5h;
|
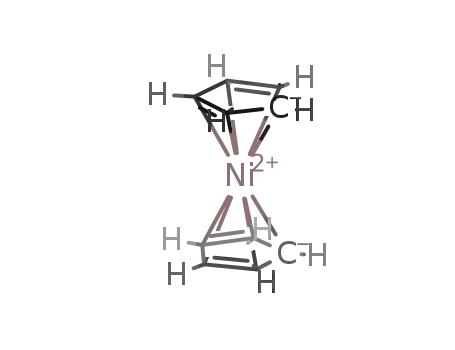
nickelocene

bis(1,5-cyclooctadiene)nickel (0)

triphenylphosphine

nickel dichloride
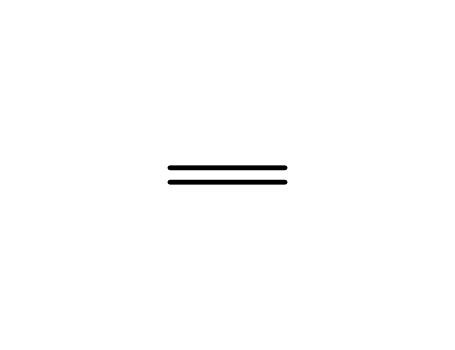
ethene
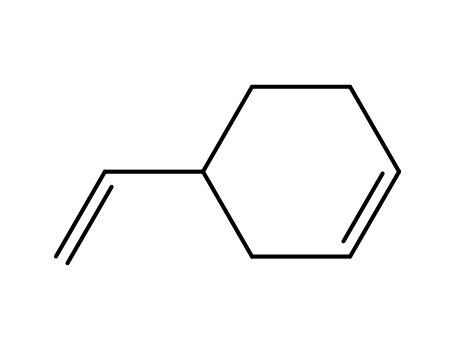
4-ethenylcyclohexene
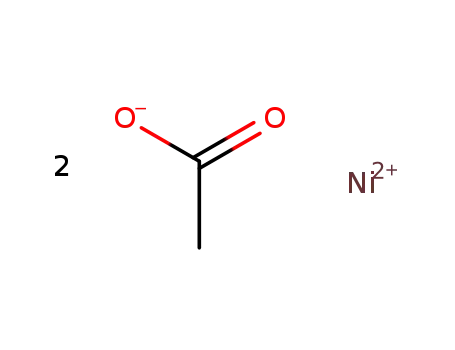
nickel diacetate
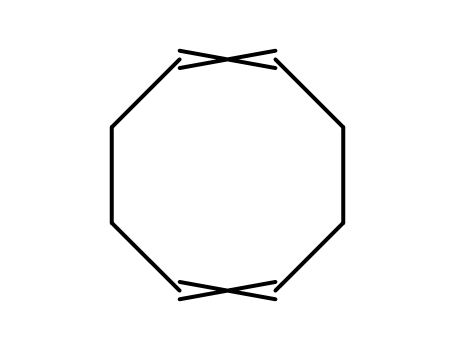
cyclo-octa-1,5-diene
CAS:1028648-22-7
Molecular Formula:C24H18BNO2
Molecular Weight:363.2
CAS:189367-54-2
CAS:16903-61-0