Your Location:Home >Products >Organic phosphines >Phenyl phosphines >184095-69-0
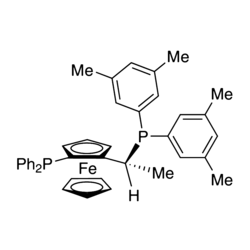

Product Details
|
Reactions |
Ligand for Rhodium-catalyzed asymmetric hydrogenation in the synthesis of tertiary carbinamide. |
|
Chemical Properties |
Orange powder |
|
General Description |
sold in collaboration with Solvias AG |
InChI:InChI=1/C35H35P2.C5H5.Fe/c1-25-19-26(2)22-32(21-25)36(33-23-27(3)20-28(4)24-33)29(5)34-17-12-18-35(34)37(30-13-8-6-9-14-30)31-15-10-7-11-16-31;1-2-4-5-3-1;/h6-24,29H,1-5H3;1-5H;/t29-;;/m0../s1
Herein we report the copper-catalyzed si...
The invention discloses a synthesis meth...
(diphenylphosphin)ferrocene

vinyl bis(3,5-dimethylphenyl)phosphine

(R)-(-)-1-[(S)-2-(diphenylphosphino)ferrocenyl]ethylbis(3,5-dimethylphenyl)phosphine
| Conditions | Yield |
|---|---|
|
With iron(III) chloride; D-Prolin; In dichloromethane; at 25 ℃; for 12h; Inert atmosphere;
|
95% |
C40H40FeP2*2BH3

C40H40FeP2
| Conditions | Yield |
|---|---|
|
With 1,4-diaza-bicyclo[2.2.2]octane; In toluene; at 90 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
(diphenylphosphin)ferrocene
CAS:15133-82-1