Your Location:Home >Products >OLED intermediates >Thiophenes >149703-84-4
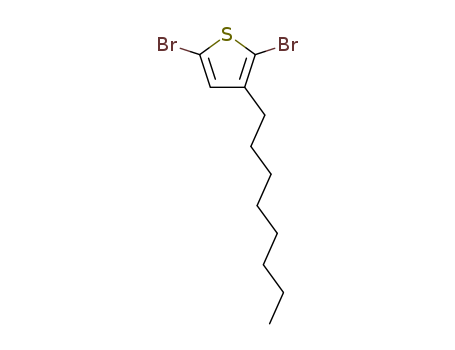

Product Details
Chemical Properties
Colorless to yellow to brown liquid
Uses
It is a conducting polymer precursor.
InChI:InChI=1/C12H18Br2S/c1-2-3-4-5-6-7-8-10-9-11(13)15-12(10)14/h9H,2-8H2,1H3
Two [thiophene-1,6-dithienylhexa-1,3,5-triene] copolymers were prepared by a palladium catalysed coupling reaction of a dibromothiophene derivative and a bis(tributylstannyl) derivative of a 1,6-dithienylhexa-1,3,5-triene unit. The electrochromism and the photoluminescence properties of the highly conjugated polymers were studied. In the solid state the polymers have strong photoluminescence bands at 2.0 eV for 9 and 1.95 eV for 8. Polymer 8 seems particularly promising for use as a red-light emitting diode, and the two polymers 8 and 9 exhibit red-pale blue electrochromism that makes them suitable for fabricating new devices.
The photophysical properties of a π-conjugated metal-organic oligomer vary smoothly with solvent composition. The variation is believed to arise from solvent-tuned configuration mixing of 3π,π* and 3MLCT levels.
A series of dialkynyl-functionalized oligo(3-octylthiophene)m (m = 1-7 and 9, where m is the number of thiophene units) were polymerized together with 2,6-bis(pyrazole)pyridine (BPP) to get alternating copolymers (P1-P7 and P9) with the number-average molecular weights (Mn) in the range of 5.3-11 kDa. These copolymers were further self-assembled into solid microspheres. The optical emission range of these copolymers were fine-tuned from blue to red and white by changing the conjugation length of thiophene oligomers (band gap approximately from 2.1 to 1.67 eV) and coordination of metals like Eu3+ and Tb3+ in both the solution and solid states. A white color was obtained in solution, thin film, and microsphere states with CIE (Commission Internationale de l'Eclairage) coordinates close to the values of standard white color.
During the past decades several synthetic routes toward the low band gap polymer poly(2,5-thienylene vinylene) (PTV) and derivatives have been studied. This study describes an extensive NMR characterization of 13C-labeled 3-octyl-PTV and its precursor polymer prepared via the dithiocarbamate route which is, since stable monomers are available, a promising route toward PTV derivatives. By introducing 13C-labeled vinylene carbons, we were able to characterize these polymers in a quantitative way, taking the end groups and structural polymerization defects, which disturb the conjugated system, into account. Several NMR techniques and the synthesis of model compounds were used to fully assign the proton and carbon chemical shifts. Moreover, the classically used thermal conversion of the precursor toward the conjugated polymer has been compared to a smoother, acid-induced elimination procedure.
Facile ways towards the integration of the regioregular poly(3-alkylthiophene)s onto carbon nanotubes, providing multifunctional materials that combine the extraordinary properties of the carbon nanotubes with those of regioregular poly(3-alkylthiophene)s, are presented.
The synthesis of, two new poly(thienylene vinylene) derivatives is described, i.e. poly(3-octyl-2,5-thienylene vinylene) (O-PTV) and poly(bis[octylphenyl-2,5-thienylene vinylene]) (BOP-PTV). Both polymers have been prepared via the dithiocarbamate (DTC) precursor route. The polymerization protocol of the monomer toward the precursor polymer has been optimized by the use of different bases, leading to improved reproducibility of the polymerization step. Processability has been guaranteed by the introduction of alkyl side chains. Finally the precursor polymers were converted toward conjugated polymers and they were fully characterized by UV/vis, IR, GPC, and cyclic voltammetry. Bulk heterojunction solar cells with PCBM as acceptor showed promising power conversion efficiencies of 0.80% for BOP-PTV and 0.92% for O-PTV.

3-octylthiophene

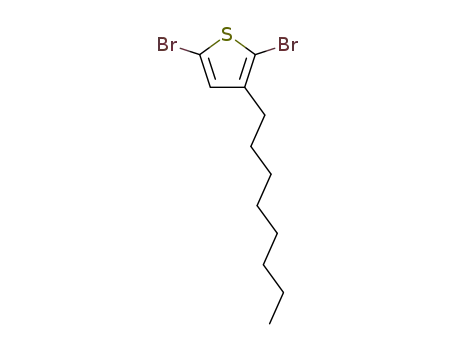
2,5-dibromo-3-octadecylthiophene
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 72h;
Darkness;
|
86% |
|
With
bromine;
In
chloroform;
at 20 ℃;
for 10h;
|
76% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 0.5h;
Heating;
|
50% |
|
With
bromine;
In
chloroform;
Heating;
|
|
|
With
bromine;
In
chloroform;
Ambient temperature;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
|
N-bromosuccinimide [NBS]

Sodium sulfite (Na2SO3)


3-octylthiophene


2,5-dibromo-3-octadecylthiophene
| Conditions | Yield |
|---|---|
|
In
N-methyl-acetamide; Succinimide;
|
88% |

3-octylthiophene
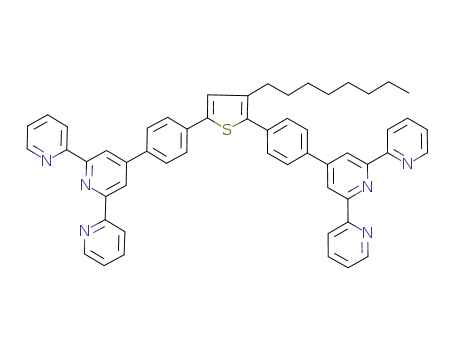
C54H46N6S
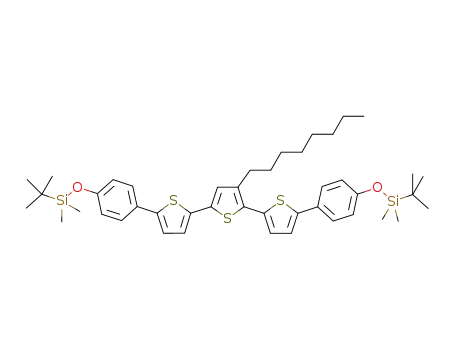
C44H60O2S3Si2

3-octylthiophene
CAS:16839-97-7
CAS:1081-34-1
CAS:145543-83-5