Your Location:Home >Products >OLED intermediates >Boric acids >13922-41-3

Product Details
|
Precautions |
Incompatible with oxidizing agents. Store in a cool, dry condition in a well sealed container. Store at room temperature. |
InChI:InChI=1/C10H9BO2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,12-13H
The oil-in-water (o/w) microemulsion con...
-
A novel 1,10-phenanthroline-containing p...
Diisopropylaminoborane (BH2-N(iPr)2) is ...
Symmetrically naphthyl-based π-conjugate...
Aryltrialkyltin compounds react with bor...
The synthesis of 2-acetyl-6-(1-naphthyl)...
Relative rates for the Lewis base-mediat...
We report the magnesiation of aryl fluor...
The invention discloses a compound takin...
Owing to the unusual reactivity of dialk...
(1-C10H7)2BNH-i-C4H9

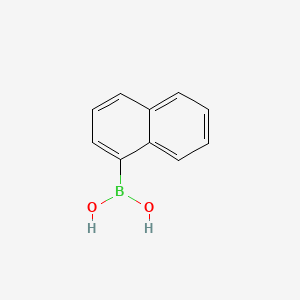
1-Naphthylboronic acid

isobutylamine
| Conditions | Yield |
|---|---|
|
With water; heating;
|
|
|
With water; heating;
|
(1-C10H7)2BNHCH3

naphthalene

1-Naphthylboronic acid
| Conditions | Yield |
|---|---|
|
With water; In water; heating;
|
|
|
With water; In water; heating;
|
(1-naphthyl)dichloroborane
triisobutyl borate
1-naphthylmagnesiumbromide
Trimethyl borate
4-(4-chloro-phenyl)-2-naphthalen-1-yl-2,3-dihydro-[1,3,5,2]oxadiazaborole
2-naphthalen-1-yl-4-(4-nitro-phenyl)-2,3-dihydro-[1,3,5,2]oxadiazaborole
2,3-dihydro-2-(naphthalen-1-yl)benzo[d][1,3,2]diazaborinin-4(1H)-one
naphthalene
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:164461-18-1