Your Location:Home >Products >OLED intermediates >Fluorenes >100124-06-9

Product Details
|
Description |
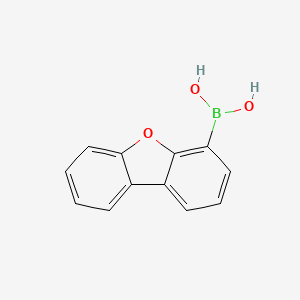
Dibenzofuran-4-boronic acid (DBFBA) is a boronic acid derivative that has been employed as a cross-coupling agent in the Suzuki coupling reaction. The Suzuki coupling reaction is a widely used method in organic synthesis for the formation of carbon-carbon bonds. Initially, DBFBA was reported to have low efficiency in the Suzuki coupling reaction. However, advances in the field, such as the use of methanol extraction and consideration of photophysical properties, have likely contributed to improvements in its application. |
| Uses | Dibenzofuran-4-boronic acid (DBFBA) is a boronic acid that has been used as a cross-coupling agent in the Suzuki coupling reaction. It has been shown to have low efficiency, but advances in this area have been made through the use of methanol extraction and photophysical properties. |
|
Chemical Properties |
off-white to beige powder |
| Isomeric SMILES | B(C1=C2C(=CC=C1)C3=CC=CC=C3O2)(O)O |
| Exact Mass | 212.0644743 g/mol |
| Monoisotopic Mass | 212.0644743 g/mol |
| Complexity | 259 |
InChI:InChI=1/C6H5BF2O2/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,10-11H
Dibenzofuran-4-boronic acid was purchased from Alfa Aesar. THF was purified by PURE SOLV (Innovative Technology) purification system. Other reactants or reagents were used as …
Increasing the boronic acid to 3.0 molar equiv … acid, use of 1.5 molar equiv of dibenzofuran-4-boronic acid resulted in a slow reaction (46 h). In this case increasing the boronic …
The boronic acid moiety is a very useful functional group for the preparation of sugar sensors. Along this line, water-soluble boronic acids that change fluorescent properties upon sugar binding are especially useful as reporter units in fluorescent sensors for carbohydrates. Herein, we report the discovery of a new water-soluble boronic acid (1, dibenzofuran-4-boronic acid) that exhibits unique fluorescence changes at three wavelengths upon binding with sugars under near physiological conditions.
However, when using the ortho-substituted o-tolylboronic acid and dibenzofuran-4-boronic acid the yields dropped significantly to 35% and 25% respectively, showing the problems …
dibenzofuran
Triisopropyl borate
4-bromodibenzofuran
Trimethyl borate
3-dibenzofuran-4-yl-2-methoxy-benzaldehyde
N-(dibenzofuran-4-yl)-N-phenyl-amine
dibenzo[b,d]furan-4-yl(phenyl)methanone
3-{1-[3-(tert-butyldiphenylsilanyloxy)-propyl]-1H-pyrrolo[2,3-b]pyridin-3-yl}-4-dibenzofuran-4-yl-pyrrole-2,5-dione
CAS:1001911-63-2
Molecular Formula:C<sub>18</sub> H<sub>14</sub> BNO<sub>2</sub>
Molecular Weight:287.1
CAS:89827-45-2