Your Location:Home >Products >OLED intermediates >Thiophenes >4805-22-5
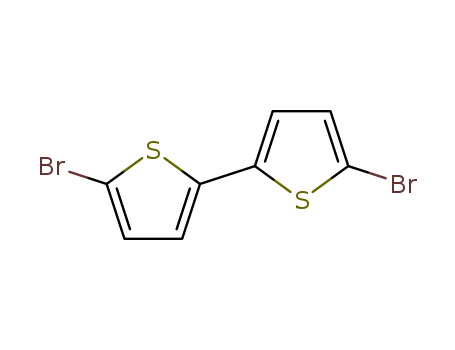

Product Details
Chemical Properties
white to light yellow shiny flakes or
Uses
It is an active pharmaceutical ingredient and an OLED (organic light-emitting diode) intermediate.
Uses
A termination reagent for polymer ization
A novel carbazole-based fluorophore 5-(carbazol-9-yl)-5′-mercapto-2, 2′-bithiophene (CBTSH) was synthesized. CBTSH exhibited emission enhancement when attached to the surface of CdSe quantum dots (QDs). The emission quantum yield of CBTSH attached to CdSe is almost 5 times larger than that of CBTSH alone. The emission enhancement of CBTSH on the CdSe surface is due to the restriction on the rotational and vibrational motions of the carbazolylbithienyl unit as a result of QD aggregation.
-
We have synthesized and characterized a series of novel compounds of α,α'-bis(aminomethyl)oligothiophenes and their related compounds whose degree of polymerization spans two (dimer) to five (pentamer). The compounds presented in our studies are α,α'-bis[(2,2,5,5-tetramethyl-1-aza-2,5-disilacyclopenthyl)methyl] oligothiophene, α,α'-bis(aminomethyl)oligothiophene dihydrochloride and α,α'- bis(aminomethyl)oligothiophene. These compounds exhibit desired chemical activity while maintaining controllable electronic properties. The synthetic processes of the oligothiophenes are as follows: 2-aminomethylthiophene is first 'protected' and the thiophene rings are coupled using standard Grignard methods. The protected groups are removed at the final stage of the reaction. The compounds show unique properties; for example α,α'-bis(aminomethyl)oligothiophene dihydrochloride is soluble in water. The results of electronic spectra and duration are also presented.
A new star-shaped D-π-A molecule with triphenylamine (TPA) as core and donor unit, octyl cyanoacetate (CA) as end group and acceptor unit, and 2,2′-bithiophene vinylene (bTV) as π bridge, S(TPA-bTV-CA) was designed and synthesized for the application as donor materials in solution-processed bulk-heterojunction organic solar cells (OSCs). The compound is soluble in common organic solvents. The thermal, optical and electrochemical properties of the star molecule were studied. The OSC devices were fabricated by spin-coating the blend solution of the molecule as donor and PC70BM as acceptor (1:3, w/w). The OSC device based on S(TPA-bTV-CA)/PC70BM demonstrated a high open circuit voltage of 0.91 V, a short circuit current density of 4.64 mA/cm2, a fill factor (FF) of 50%, corresponding to a power conversion efficiency of 2.1%, under the illumination of AM 1.5, 100 mW/cm 2.
The 6,6′-([2,2′:5′,2″:5″,2-quaterthiophene]-5,5-diyl)bis(1,3,5-triazine-2,4-diamine) (MR100), has been previously investigated in our research group through its biological activities toward pathogenic prion proteins (PrPSc). This compound presents a high affinity to protein strains and interacts selectively with at least one β-sheet rich isoform of prion protein. Herein we present the improved total synthesis of MR100, through a palladium-catalyzed direct double arylation using the concerted metalation deprotonation mechanism (CMD).
2-Bromo-2′-phenyl-5,5′-thiophene was synthesized by cross-coupling reaction of phenylboric acid and 2,2′-dibromo-5,5′-bithiophene with a Suzuki reaction; we found the Suzuki reaction to give a higher yield when compared to the Negishi reaction.
A chiral oligothiophene, possessing in-chain chirality, was prepared and its racemate was characterized by single crystal X-ray crystallography; the in-chain chiral 1,1′-binaphthyl moiety interrupts the π-conjugation and affects the solid state properties
The invention discloses a synthesis method of aryl halides (including aryl bromide shown as a formula (2) and aryl iodide shown as a formula (3)). All the systems are carried out in an air atmosphere,visible light is utilized to excite a substrate or a photosensitizer to catalyze the reaction; and in a reaction solvent, when aromatic hydrocarbon shown in the formula (1) and sodium bromide serve as raw materials, aryl bromide shown in the formula (2) is obtained through a reaction under the auxiliary action of an additive (protonic acid); or when aromatic hydrocarbon shown in the formula (1) and sodium iodide are used as raw materials, under the auxiliary action of an additive (protonic acid), aryl iodide shown in the formula (3) is obtained through reaction. The synthesis method has the advantages of cheap and accessible raw materials, simple reaction operation and mild reaction conditions. The method is compatible with the arylamine which is liable to be oxidized. The invention provides a new method for the synthesis of aryl halides, realizes the amplification of basic chemicals aryl halides including aryl bromide shown in the formula (2) and aryl iodide shown in the formula (3),and has wide application prospect and practical value.
Organic halides are critical building blocks that participate in various cross-coupling reactions. Furthermore, they widely exist as natural products and artificial molecules in drugs with important physiological activities. Although halogenation has been well studied, to the best of our knowledge, studies focussing on sensitive systems (e.g.aryl amines) have not been reported. Herein, we describe a compatible oxidative halogenation of (hetero)arenes with air as the oxidant and halide ions as halide sources under ambient conditions (visible light, air, aqueous system, room temperature, and normal pressure). Moreover, this protocol is practically feasible for gram-scale synthesis, showing potential for industrial application.
Substituted bithiophenes are prominent fragments in functional organic materials, and they are ideally prepared via direct oxidative C-H/C-H coupling. Here, we report a novel PdII catalyst system, employing 1,10-phenanthroline-5,6-dione (phd) as the ancillary ligand, that enables aerobic oxidative homocoupling of 2-bromothiophenes and other related heterocycles. These observations represent the first use of phd to support Pd-catalyzed aerobic oxidation. The reaction also benefits from a Cu(OAc)2 cocatalyst, and mechanistic studies show that Cu promotes C-C coupling, implicating a role for CuII different from its conventional contribution to reoxidation of the Pd catalyst.
In this work, we present a series of newly synthesized conjugated oligothiophene derivatives, with different numbers of central thiophene units, and different donor/acceptor architectures. Electrochemical and spectroscopic data have also been reported. We used thiophene or bithiophene as central donor core units, 3-octylthiophenes as π-bridge and solubilizing sub-units, and ethyl cyanoacetate or rhodanine moieties as acceptor end groups, in order to get D-π-A and A-π-D-π-A molecular architectures. The length of the synthesized oligothiophenes ranges from three to eight thiophene units, a variety that is sufficient to put in evidence different optical and electrochemical characteristics as well as semiconducting characteristics. Oligothiophene compounds can be regarded not only as models for the study of structure-property relationships relative to polythiophenes, but also they present a large number of applications in the field of organic electronics (i.e.: as donors in bulk-heterojunction solar cells and hole-transporting layer materials in perovskite solar cells, among others).
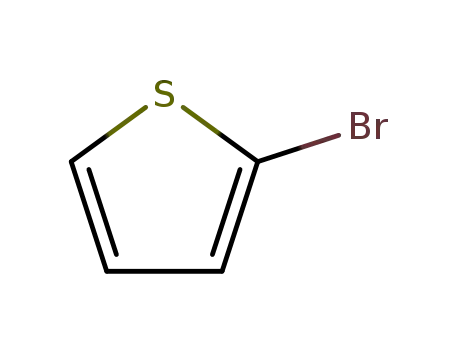
2-bromothiophene

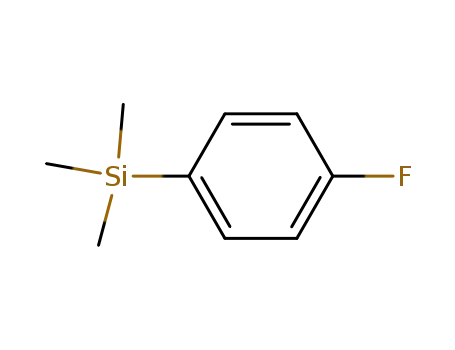
(p-fluorophenyl)trimethylsilane

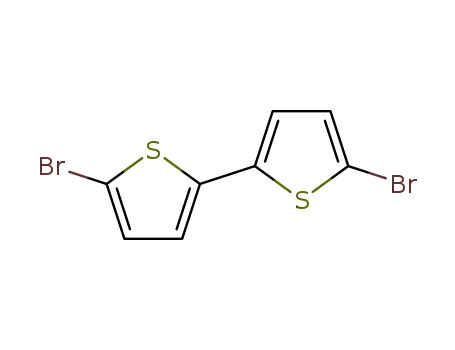
5,5'-dibromo-2,2'-bisthiophene

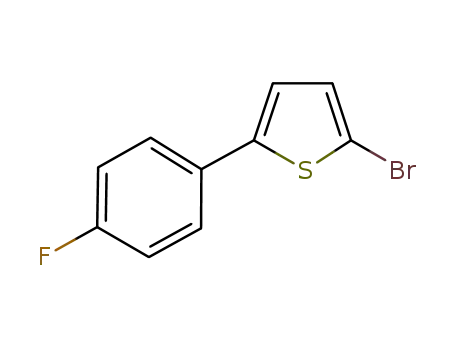
2-bromo-5-(4-fluorophenyl)thiophene

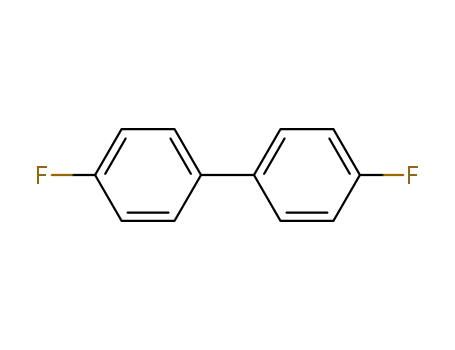
4,4'-difluorobiphenyl
| Conditions | Yield |
|---|---|
|
With
gold(lll) acetate; (1S)-10-camphorsulfonic acid;
In
chloroform-d1; d(4)-methanol;
at 25 ℃;
for 2h;
|
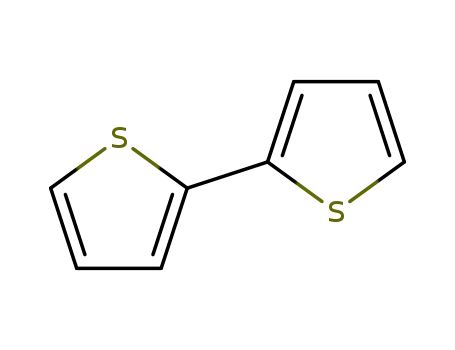
2,2'-Bithiophene


5,5'-dibromo-2,2'-bisthiophene
| Conditions | Yield |
|---|---|
|
With
mono(N,N,N-trimethylbenzenaminium) tribromide;
zinc(II) chloride;
In
acetic acid;
for 27h;
Ambient temperature;
|
99% |
|
With
N-Bromosuccinimide; acetic anhydride;
|
99% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 0 ℃;
for 0.5h;
Inert atmosphere;
|
99.8% |
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 20 ℃;
for 0.05h;
Ultrasonic irradiation;
|
98% |
|
With
N-Bromosuccinimide; acetic acid;
In
chloroform;
at 70 ℃;
for 4h;
|
98% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
at 70 ℃;
for 4h;
|
95.1% |
|
With
2-bromo-3,4-dichloropyridazin-3(2H)-one; zinc dibromide;
In
dichloromethane;
at 20 ℃;
for 0.0833333h;
regioselective reaction;
|
94% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 8h;
|
93% |
|
With
N-Bromosuccinimide;
In
chloroform; acetic acid;
for 6h;
Reflux;
Inert atmosphere;
|
93% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 2h;
Reflux;
|
93% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 - 20 ℃;
Inert atmosphere;
|
92% |
|
With
bromine; acetic acid;
at 30 ℃;
for 2h;
|
90% |
|
With
N-Bromosuccinimide; acetic acid;
at 20 ℃;
for 3h;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
Cooling with ice;
|
90% |
|
With
N-Bromosuccinimide;
In
acetone;
at 20 - 60 ℃;
|
90% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
|
88% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 3 - 8 ℃;
|
87.5% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
85% |
|
With
N-Bromosuccinimide;
|
85% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 3h;
|
85.69% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
84% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at -20 ℃;
for 4h;
|
80% |
|
With
bromine; sodium hydrogencarbonate;
In
chloroform;
for 1h;
Ambient temperature;
|
77% |
|
With
dihydrogen peroxide; ammonium bromide; acetic acid;
In
water;
for 16h;
Time;
Solvent;
Reagent/catalyst;
regioselective reaction;
Green chemistry;
|
76% |
|
With
n-butyllithium; bromine;
In
tetrahydrofuran;
at -78 ℃;
|
60% |
|
With
water; sodium bisulfate hydrate; sodium bromide;
In
acetonitrile;
for 96h;
Irradiation;
|
57% |
|
With
sodium hydrogensulfate monohydrate; water; sodium bromide;
In
acetonitrile;
at 20 ℃;
for 96h;
Schlenk technique;
Irradiation;
Green chemistry;
|
57% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 0 ℃;
for 12h;
|
51% |
|
With
lead(IV) tetraacetate; lithium bromide;
In
chloroform;
at 20 ℃;
for 4h;
regioselective reaction;
|
28% |
|
With
N-Bromosuccinimide;
In
acetic acid;
|
|
|
With
N-Bromosuccinimide;
In
methanol;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
|
|
With
N-Bromosuccinimide;
|
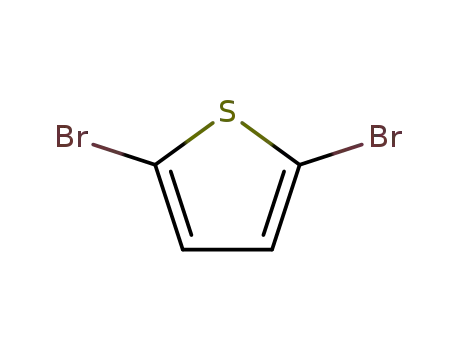
2,5-dibromothiophen

2,2'-Bithiophene
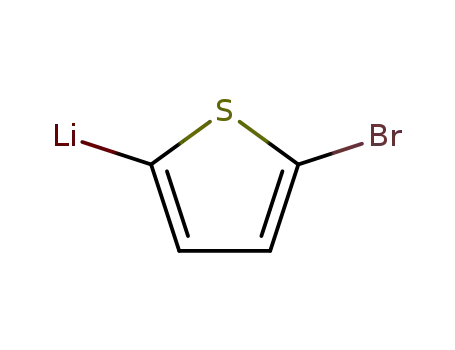
5-bromo-[2]thienyl lithium

2-bromothiophene
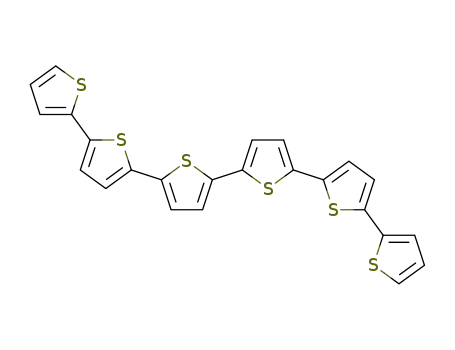
sexithiophene
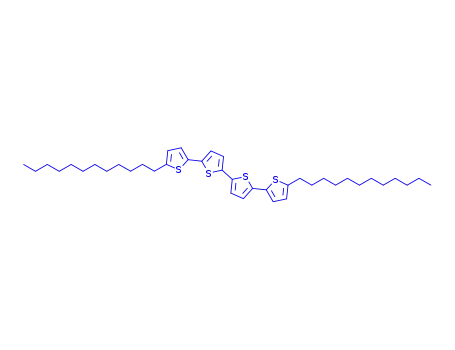
5,5'''-didodecyl-2,2':5',2'':5'',2'''-quaterthiophene
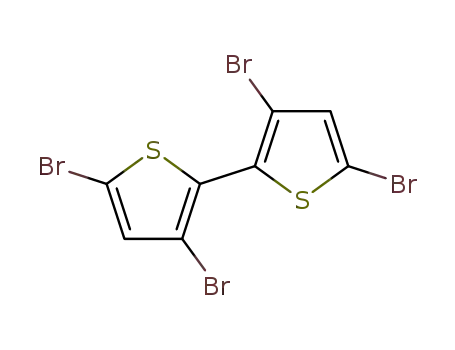
3,3',5,5'-tetrabromo-2,2'-bithiophene

3,3?-didodecyl-2,2′;5′,2″;5″,2?-quaterthiophene
CAS:1002345-50-7
Molecular Formula:C27H52B2F8P2
Molecular Weight:612.3
CAS:1795-01-3