Your Location:Home >Products >OLED intermediates >Thiophenes >17573-92-1

Product Details
Chemical Properties
Clear light brown liquid
Synthesis Reference(s)
Synthetic Communications, 20, p. 213, 1990 DOI: 10.1080/00397919008052285
General Description
3-Methoxythiophene is a thiophene. The intramolecular and intermolecular geometries of crystals of 3-methoxythiophene were studied. Spectroscopic studies of bipolarons derived from oligomerized 3-methoxythiophene in solution has been reported. The electropolymerization of 3-methoxythiophene on Pt and Fe electrodes in an aqueous micellar medium containing sodium dodecyl sulfate and 10-3M bithiophene has been reported. Thin polymer films of 3-methoxythiophene at the cathode in a direct current discharge have been prepared.
InChI:InChI=1/C5H6OS/c1-6-5-2-3-7-4-5/h2-4H,1H3
A method for the Bronsted acid promoted desulfination of aryl sulfoxides is presented. In the presence of a thiol, electron-rich sulfoxides undergo C-S bond cleavage to give the corresponding protodesulfinated arenes and disulfides.
A series of organometallic complexes [Cl(dppe)2Ru?C≡C-(3-R?C4H2S)-C≡C?Ru(dppe)2Cl] (3-R-C4H2S=3-substituted thienyl moiety; R=?H, ?C2H5, ?C3H7, ?C4H9, ?C6H13, ?OMe, ?CN in 5 a–5 g respectively) have been synthesized by systematic variation of 3-substituents at the thienylethynyl bridging unit. The diruthenum(II) wire-like complexes (5 a–5 g) have been achieved by the reaction of thienylethynyl bridging units, HC≡C-(3-R-C4H2S)-C≡CH (4 a–4 g) with cis-[Ru(dppe)2Cl2]. The wire-like diruthenium(II) complexes undergo two consecutive electrochemical oxidation processes in the potential range of 0.0 - 0.8 V. Interestingly, the wave separation between the two redox waves is greatly influenced by the substituents at the 3-position of the thienylethynyl. Thus, the substitution on 3-position of the thienylethynyl bridging unit plays a pivotal role for tuning the electronic properties. To understand the electronic behavior, density functional theory (DFT) calculations of the selected diruthenium wire-like complexes (5 a–5 e) with different alkyl appendages are performed. The theoretical data demonstrate that incorporation of alkyl groups to the thienylethynyl entity leaves unsymmetrical spin densities, thus affecting the electronic properties. The voltammetric features of the other two Ru(II) alkynyl complexes 5 f and 5 g (with ?OMe and ?CN group respectively) show an apparent dependence on the electronic properties. The electronic properties in the redox conjugate, (5 a+) with Kc of 3.9×106 are further examined by UV-Vis-NIR and FTIR studies, showing optical responses in NIR region along with changes in “?Ru?C≡C?“ vibrational stretching frequency. The origin of the observed electronic transition has been assigned based on time-dependent DFT (TDDFT) calculations.
We demonstrated herein the facile synthesis of triisopropylsilylethynyl (TIPS) functionalized benzo[1,2-b:4,5-b′]dithiophene (BDT). Three new TIPSBDT-based donor-acceptor alternating copolymers were further developed by Pd-catalyzed Stille coupling. The effect of accepting unit structure on the optical, electrochemical and energy levels of the polymer was studied. The positive impact of PFN layer, high-boiling solvents processing, polar solvent treatment and solvent annealing on the performance of polymer:PC71BM bulk heterojunction solar cells was revealed. The best devices delivered a power conversion efficiency of 5.46% when blend films processed using o-dichlorobenzene with 3 vol% DIO and treated with the optimization of THF annealing and insertion of PFN layer. The device performance was correlated with the morphology evolution of blend films processed with solvent choice, methanol treatment and THF annealing.
A ligand-free, powerful, and practical method for mono and polymethoxylation of unactivated aryl bromides has been developed; CuCl was used as catalyst, HCOOMe as cocatalyst, and methanolic MeONa as both nucleophile and solvent. This eco-friendly procedure is characterized by operational simplicity, inexpensive substrates (unactivated mono to polybromoarenes), full conversion, and direct recovery of pure MeOH.
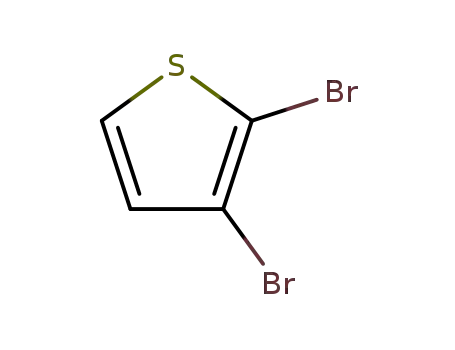
2,3-dibromothiophen

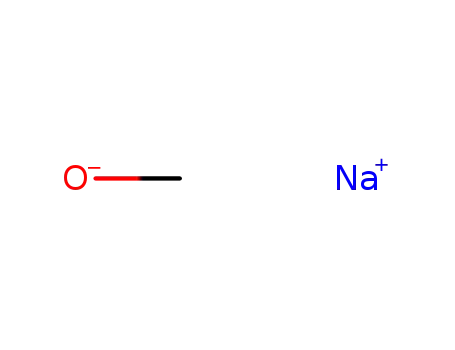
sodium methylate

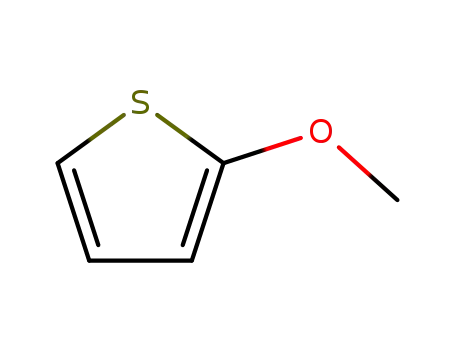
2-methoxythiophene

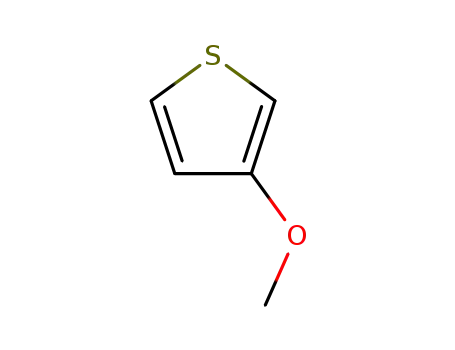
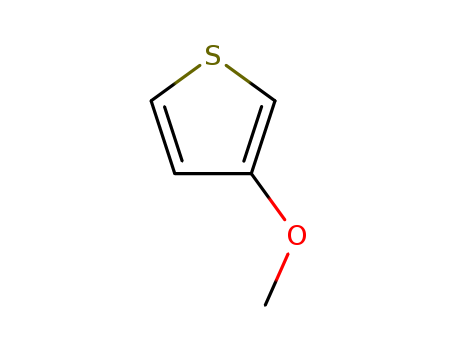
3-methoxy-thiophene

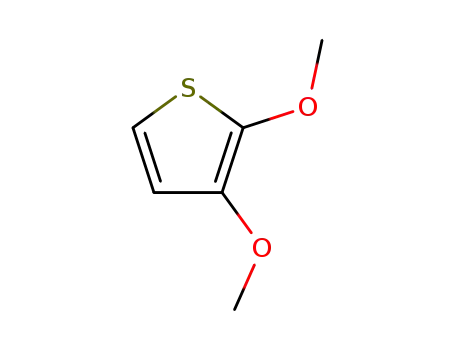
2,3-dimethoxythiophene
| Conditions | Yield |
|---|---|
|
copper(I) bromide;
In
methanol;
at 97 ℃;
for 6h;
|
30% 60% 7% |
|
copper(I) bromide;
In
methanol;
at 97 ℃;
for 6h;
|
30% 7% 60% |

2,3-dibromothiophen


sodium methylate

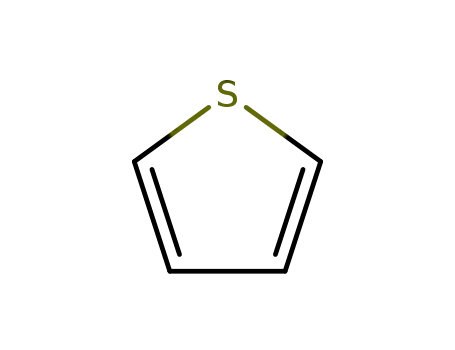
thiophene


2-methoxythiophene


3-methoxy-thiophene


2,3-dimethoxythiophene
| Conditions | Yield |
|---|---|
|
copper(I) bromide;
In
methanol;
at 97 ℃;
for 6h;
Product distribution;
Mechanism;
also reaction of 2,4-, 2,5- and 3,4-dibromothiophene;
|
30% 7% 60% 3% |
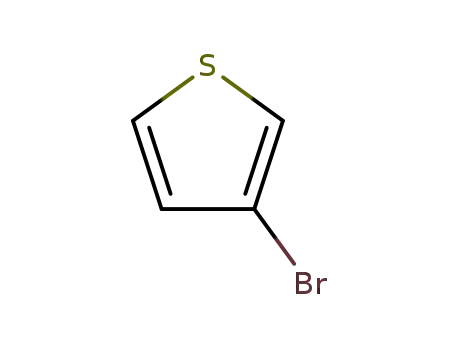
3-Bromothiophene

sodium methylate
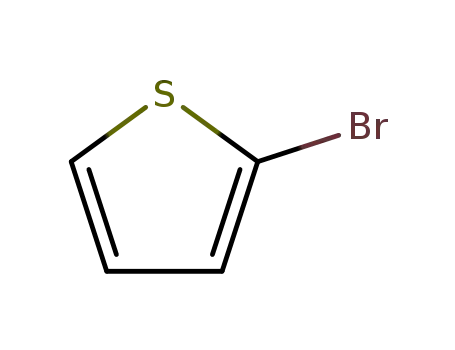
2-bromothiophene

2,3-dibromothiophen
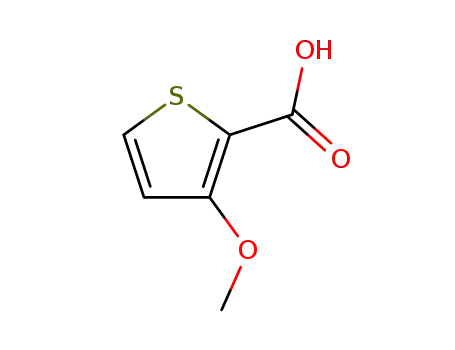
3-methoxythiophene-2-carboxylic acid
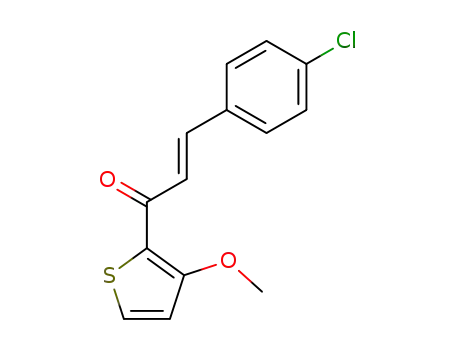
E-1-(3-Methoxy-2-thienyl)-3-(4-chlorphenyl)-2-propen-1-on
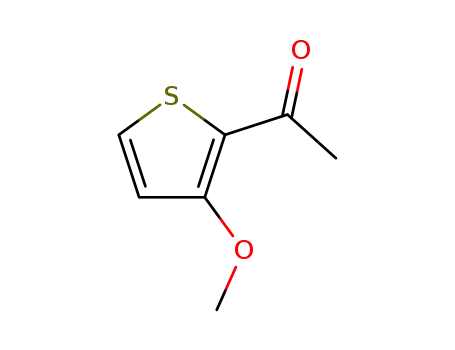
2-acetyl-3-methoxythiophene
(3-methoxy-2-thienyl)phenylmethanol
CAS:577-19-5
CAS:16839-97-7
CAS:932-41-2