Your Location:Home >Products >OLED intermediates >Thiophenes >932-41-2
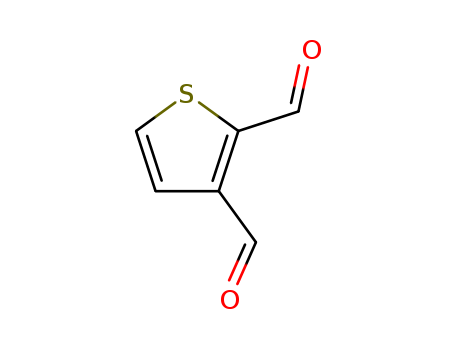

Product Details
Chemical Properties
brown powder
Uses
2,3-Thiophenedicarboxaldehyde may be employed for the synthesis of TIPSE (triisopropylsilylethynyl)-substituted dibromoanthradithiophene (TIPSEBr2- AntDT).
General Description
2,3-Thiophenedicarboxaldehyde undergoes aldol reaction with (4aS,9R,9aR,10S)-11-(propan-2-ylidene)-2,3,4a,9,9a,10-hexahydro-9,10-methanoanthracene-1,4-dione under oxygen-free conditions affords (5aS,6S,11R,11aR)-14-(propan-2-ylidene)-5a,6,11,11a-tetrahydro-6,11-methanotetraceno[3,2-b]thiophene-5,12-dione.
InChI:InChI=1/C6H4O2S/c7-3-5-1-2-9-6(5)4-8/h1-4H
Treatment of cyclic α-nitroketones and aromatic 1,2-dialdehydes with DBU in tetrahydrofuran containing small amounts of water proceeded through two chemodivergent one-pot domino pathways, whose outcome depended on the ring size of the starting nitroketone. Thus, α-nitrocyclohexanone underwent diastereoselective α′-arylmethylenation reactions through a nitroaldol/aldol/reverse nitroaldol mechanism. On the other hand, α-nitrocycloheptanone and α-nitrocyclooctanone afforded 2-nitroindane-1,2-diols containing three contiguous stereocenters in a highly diastereoselective fashion through a nitroaldol/retro-Dieckmann/intramolecular nitroaldol process.
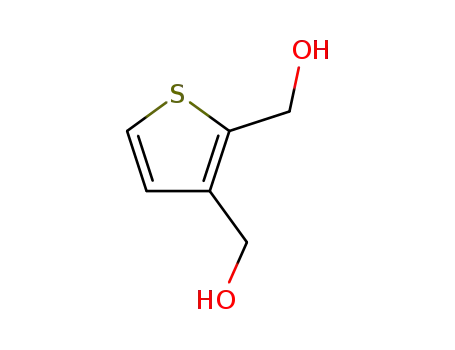
2,3-bis(hydroxymethyl)thiophene

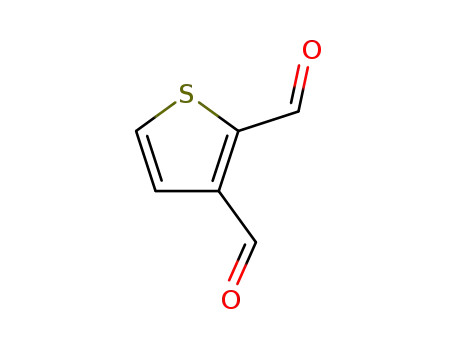
2,3-diformylthiophene
| Conditions | Yield |
|---|---|
|
With
1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione;
In
ethyl acetate; acetone;
at 80 ℃;
for 3.5h;
|


2,3-diformylthiophene
| Conditions | Yield |
|---|---|
|
|

2,3-bis(hydroxymethyl)thiophene
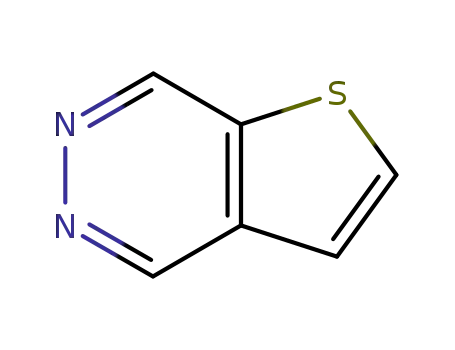
Thieno<2,3-d>pyridazine
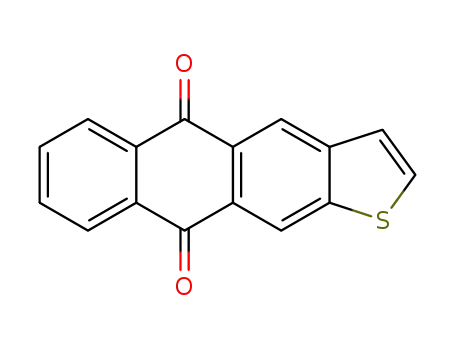
Anthra<2,3-b>thiophene-5,10-dione
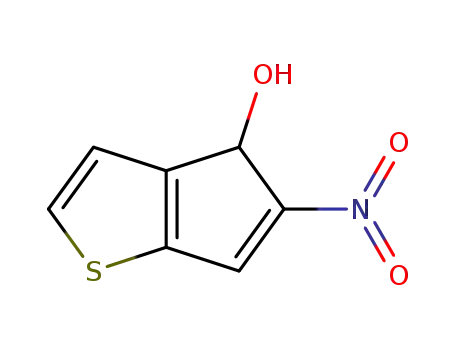
5-nitro-4H-cyclopentathiophen-4-ol
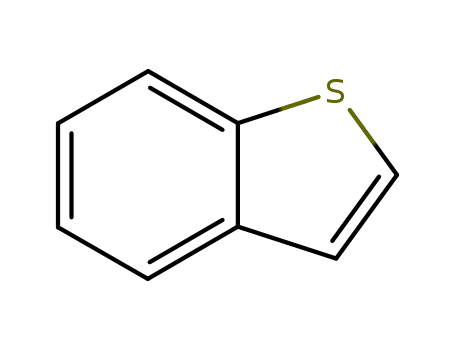
Benzo[b]thiophene
CAS:855-38-9
CAS:2618-96-4
CAS:17573-92-1
CAS:51792-34-8