Your Location:Home >Products >OLED intermediates >Thiophenes >51792-34-8
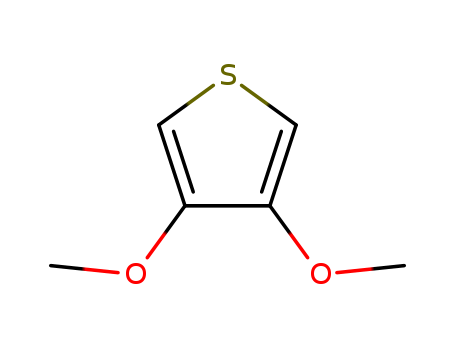

Product Details
Chemical Properties
Colorless to yellow to orange liquid
Uses
3,4-Dimethoxythiophene is used as an electronic materials intermediate. It is used as a starting material in the synthesis of porphyrin dyad which is used to study photoinduced energy transfer.
Uses
Building block in the synthesis of an N2S2-N4 porphyrin dyad used to study photoinduced energy transfer.
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 2956, 1951 DOI: 10.1021/ja01150a525
General Description
3,4-Dimethoxythiophene (DMOT) is a monomer and a precursor which can be synthesized by ring closure reaction of 2,3-dimethoxy-1,3-butadiene and sulfur dichloride in hexane medium. It is an oligothiphene that is majorly used in the development of electroactive materials for organic electronics based applications.
InChI:InChI=1/C6H8O2S/c1-7-5-3-9-4-6(5)8-2/h3-4H,1-2H3
-
A number of 3,4-dialkoxythiophene compounds have been synthesised and their reactivities assessed via the Mannich reaction with secondary amines. These reactions surprisingly gave the bis-Mannich bases substituted at the 2- and 5-positions as well as the expected mono-Mannich bases substituted at the 2-position. Conditions were optimised to give symmetrical bis-2,5-Mannich bases in one step and asymmetrical bis-2,5-Mannich bases in two steps. Several bis(thien-2-ylmethyl)amines derived from 3,4-dialkoxythiophenes are reported, their synthesis being performed under both normal and high dilution conditions. Some syntheses also afforded the (thien-2-ylmethyl)amine oligomers. Further substitution of the bis(thien-2-ylmethyl)amines at the 5-position via the Mannich reaction also proved successful. The factors affecting the yields and substitution patterns are discussed, together with molecular modelling of the spatial requirements.
The asymmetrical sulfur analog of 3,4-ethylenedioxythiophene (EDOT), thieno[3,4-b]-1,4-oxathiane (EOTT), was synthesized, and its electropolymerization was comparatively investigated by employing different solvent-electrolyte systems (room temperature ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate (BmimPF6), CH2Cl2-Bu4NPF6, and CH2Cl2-BmimPF6). Further, the effect of solvents and supporting electrolytes on the structure, morphology, electrochemical, electronic, and optical properties and electrochromic performance of the obtained poly(thieno[3,4-b]-1,4-oxathiane) (PEOTT) films were minutely studied. PEOTT film with a band gap (Eg) of about 1.6 eV could be facilely electrodeposited in all the solvent-electrolytes and displayed excellent electroactivity, outstanding redox stability in a wide potential window, and improved thermal stability. Cyclic voltammetry showed that EOTT could be electropolymerized at a lower oxidation potential in BmimPF6 (~1.0 V vs Ag/AgCl) due to several advantanges of RTIL BmimPF6 itself, such as high intrinsic conductivity and mild chemical conditions, etc., and the resulting PEOTT film exhibited compact morphology with better electroactivity and stability and higher electrical conductivity. On the other hand, PEOTT films from all the sovent-electrolytes also showed the electrochromic nature by color changing from gray blue to green, and further kinetic studies revealed that PEOTT had decent contrast ratios (36%), higher coloration efficiencies (212 cm2/C in BmimPF6), low switching voltages, moderate response time (1.2 s), excellent stability, and color persistence. From these results, PEOTT provides more plentiful electrochromic colors and holds promise for display applications.
-
The invention discloses a 3, 4 - dimethoxy thiophene synthesis method, the method will be 2, 5 - dicarboxylic acid dimethyl ester - 3, 4 - dimethoxy thiophene dissolved in dimethyl formamide in aqueous solution, then adding the pectins catalyst, at a temperature of 130 - 180 °C, pressure is 0 - 10 kg/cm2 Reaction (table) 5 - 18 h after, filtered, pressure reducing rectification to get 3, 4 - dimethoxy thiophene, wherein filtering and rectification are in thermal insulation a pressure maintaining state. The method utilizes the pectins technology, has eliminated saponification, acidified and other intermediate links, shortens the synthetic route, reduces the consumption of raw materials, in particular reduces the production of waste aqueous solution, reduces the integrated cost, simplify the post-processing procedure, the whole synthetic route more environment-friendly, safer, more efficient. (by machine translation)
The invention relates to a method for synthesizing 3,4-dimethoxythiophene. The method comprises steps as follows: S1, 2,5-dimethyl dicarboxylate-3,4-sodium thiophene diol and dimethyl sulfate are subjected to a heating reaction in an organic solvent, and 3,4-dimethoxythiophene-2,5-dimethyl dicarboxylate is obtained; S2, 3,4-dimethoxythiophene-2,5-dimethyl dicarboxylate is subjected to the heatingreaction with strong base in an alkylbenzene organic solvent with the boiling point not lower than 200 DEG C, then strong acid is added for acidification, and 3,4-dimethoxythiophene-2-formic acid is obtained; S3, 3,4-dimethoxythiophene-2-formic acid is subjected to a reduced-pressure heating reaction in a long-chain amine organic solvent with the boiling point not lower than 300 DEG C, and 3,4-dimethoxythiophene is obtained. The method is low in production cost, low in energy consumption and high in product yield, the organic solvents can be recycled, particularly, no catalyst is used in the step of decarboxylation, so that the cost is greatly reduced, and environmental pollution is reduced.
One class of blue thiophene electrochromic compounds include 3,4-(2,2-bis(2-oxo-3-phenylpropyl))propylenedioxythiophene, 3,4-(2,2-bis(2-oxo-3-phenylbutyl))propylenedioxythiophene, and 3,4-(2,2-bis(2-oxo-3-phenylamyl))propylenedioxythiophene. The thiophene electrochromic compounds can change color between blue and transparency. The thiophene compounds can be electropolymerized on the surface of the ITO glass to form a film. The film has characteristics of low driving voltage (within ±1V), fast response time, and large transmittance difference between colored-state and bleached-state (up to 77.5%). The thiophene electrochromic compounds can be used in the electrochromic window, rearview mirror, electrochomeric display, and the like.
[Purpose] thiophene for preparing derivatives method, among other things, purity thiophene derivatives obtained in high yield by provides a method. [Constitution] halogenated thiophene and an alkali metal alkoxide seed alcohol solvent during is a process, alcohol solvent is regulated so as to have reaction outflow by including for a manipulation type well. , dialkoxy thiophene which comprises the step of obtaining an, oxygen atoms and thiophene dialkoxy said including compound or the like, an evacuating product caused by reaction while type well. outflow reaction to reaction step of reacting a carboxylic acid component characterized by including, of thiophene derivatives is manufacturing method. (by machine translation)
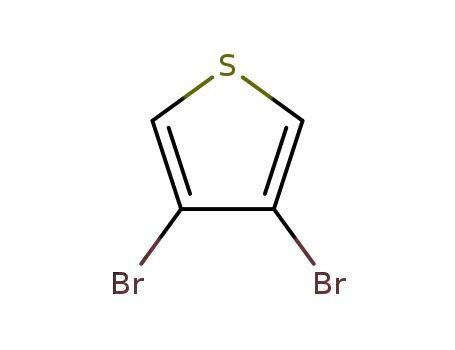
3,4-dibromothiophene

sodium methylate

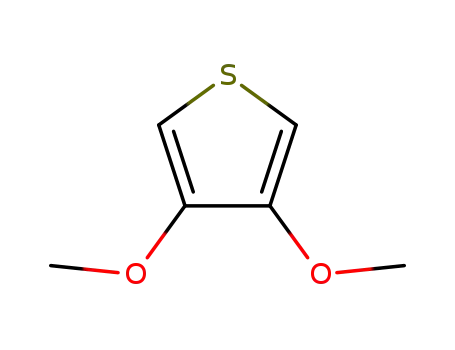
2,3-dimethoxythiophene
| Conditions | Yield |
|---|---|
|
With
potassium iodide; copper(II) oxide;
Reflux;
|
88.02% |
|
With
potassium iodide; copper(II) oxide;
In
methanol;
for 96h;
Inert atmosphere;
Reflux;
|
86.9% |
|
With
copper(I) bromide;
In
methanol;
at 70 - 97 ℃;
Temperature;
Inert atmosphere;
|
81.5% |
|
copper(I) bromide;
In
methanol;
for 6h;
Heating;
|
74% |
|
With
potassium iodide; copper(II) oxide;
In
methanol;
at 95 ℃;
for 72h;
Inert atmosphere;
|
70% |
|
With
copper(l) iodide;
In
methanol;
at 80 ℃;
for 72h;
|
60% |

3,4-dibromothiophene


2,3-dimethoxythiophene
| Conditions | Yield |
|---|---|
|
methanol;
With
sodium;
at 0 ℃;
3,4-dibromothiophene;
With
potassium iodide; copper(II) oxide;
In
methanol;
for 72h;
Heating / reflux;
|
88% |

diazomethane
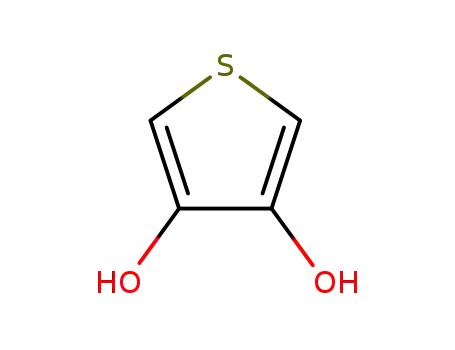
thiophene-3,4-diol
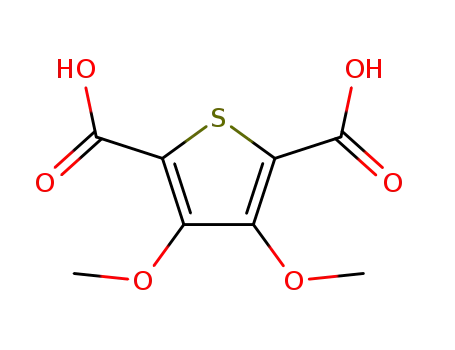
3,4-dimethoxythiophene-2,5-dicarboxylic acid

3,4-dibromothiophene
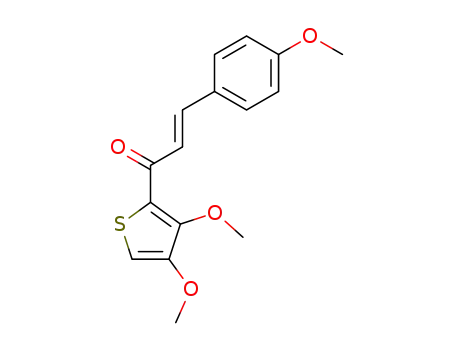
E-1-(3,4-Dimethoxy-2-thienyl)-3-(4-methoxyphenyl)-2-propen-1-on
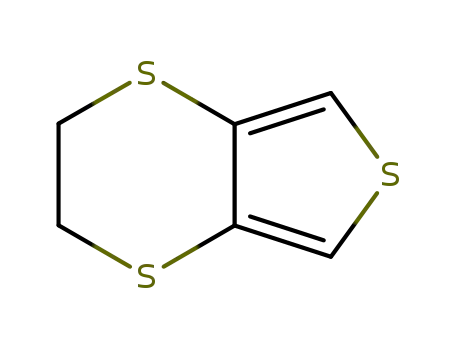
3,4-ethylenedisulfanylthiophene
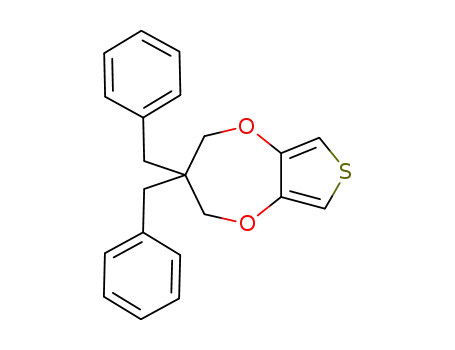
3,3-dibenzyl-3,4-dihydro-2H-thienopheno[3,4-b][1,4]dioxepine
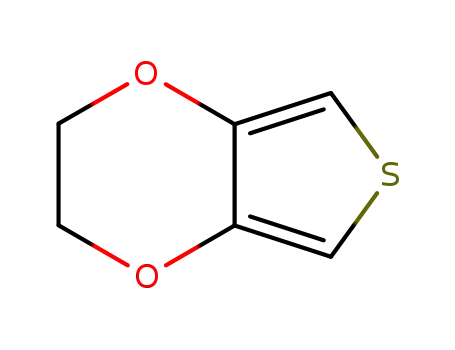
3,4-(ethylenedioxy)thiophene
CAS:1259280-37-9
CAS:657408-07-6
Molecular Formula:C26H35O2P
Molecular Weight:410.5
CAS:932-41-2
CAS:1795-01-3