Your Location:Home >Products >OLED intermediates >Carbazoles >6825-20-3

Product Details
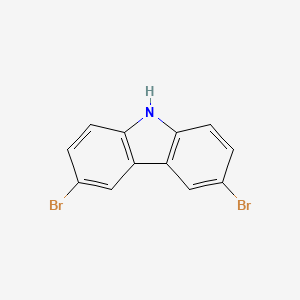
| Description | 3,6-Dibromocarbazole is a member of biphenyls and a member of bromobenzenes. |
|
Uses |
3,6-dibromocarbazole (3,6-DBCZ) is used as a pharmaceutical intermediate, and also an important intermediate of synthesizing optoelectronic materials. It has been used in the preparation of N-(2-hydroxyethyl)-3,6-dibromocarbazole. |
|
Synthesis |
3, 6-dibromocarbazole is synthesized with three methods, such as N-bromosuccinimide method, liquid bromine method and silica gel method. But now main method for synthesis is silica gel method. The main raw materials used in the synthesis include carbazole, N-bromosuccinimide, solvent methylene chloride, catalyst silica gel, and the yield could reach 89.5%. |
Isomeric SMILES: C1=CC2=C(C=C1Br)C3=C(N2)C=CC(=C3)Br
InChIKey: FIHILUSWISKVSR-UHFFFAOYSA-N
InChI: InChI=1S/C12H7Br2N/c13-7-1-3-11-9(5-7)10-6-8(14)2-4-12(10)15-11/h1-6,15H
2,7-Dibromocarbazole (2,7-DBCB) and 3,6-dibromocarbazole (3,6-DBCB) are emerging environmental pollutants, being potentially high risks to human health. In this study, interactions of the two compounds with human serum albumin (HSA) and bovine serum albumin (BSA) were investigated by molecular modeling, density functional theory calculations (DFT) and multispectral techniques.
This study investigated the toxic effects of polystyrene microplastics (PS-MPs) and 3,6-dibromocarbazole (3,6-DBCZ) on zebrafish embryos by individual/combined exposure. This study showed that individual or combined exposure of PS-MPs (10 mg/L) and 3,6-DBCZ (0.5 mg/L) could significantly increase the rate of zebrafish embryo deformity, whereas no significant effect was observed on mortality and hatching rate.
N-(4,4′-dibromodiphenylamino)-2,4,6-trimethylpyridinium tetrafluoroborate

4-bromo-N-(4-bromophenyl)-2-chloroaniline

3,6-dibromo-9H-carbazole

bis(4-bromophenyl)amine
| Conditions | Yield |
|---|---|
|
With sodium chloride; In acetonitrile; Photolysis;
|
6.8 mg |
9H-carbazole

3,6-dibromo-9H-carbazole
| Conditions | Yield |
|---|---|
|
With N-Bromosuccinimide; In tetrahydrofuran; at 85 ℃; for 48h;
|
98% |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 20 ℃;
|
97% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2.5h;
|
97% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h;
|
97% |
|
With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; In ethanol; at 20 - 25 ℃; for 2h;
|
96% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 ℃; for 24h;
|
96% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; Inert atmosphere; Schlenk technique;
|
96% |
|
With N-Bromosuccinimide; In ethyl acetate; at 0 - 20 ℃; for 5h; Inert atmosphere; Schlenk technique; Darkness; Sealed tube;
|
95% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 ℃; for 2h;
|
93% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; toluene; for 0.5h; Cooling with ice; Inert atmosphere;
|
92% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 5h;
|
91% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃;
|
91% |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 20 ℃;
|
90% |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 20 ℃;
|
90% |
|
With N-Bromosuccinimide; In dichloromethane; for 4h;
|
90% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 ℃;
|
90% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 24h;
|
88% |
|
With silica gel; In dichloromethane; at 20 ℃; Darkness;
|
87% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃;
|
86% |
|
With N-Bromosuccinimide; In dichloromethane; at 20 ℃; for 6h; Inert atmosphere;
|
85% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 6h; Flash photolysis;
|
85% |
|
With N-Bromosuccinimide; In tetrahydrofuran; at 20 ℃; for 4h;
|
83% |
|
With N-Bromosuccinimide; In tetrahydrofuran; at 20 ℃; for 4h;
|
83% |
|
With N-Bromosuccinimide; In dichloromethane; for 12h; Cooling with ice; Darkness;
|
83% |
|
With Oxone; copper(ll) bromide; In acetonitrile; at 20 ℃; for 5h; regioselective reaction;
|
82% |
|
With bromine; In chloroform; at 0 - 20 ℃; for 13h;
|
82% |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 20 ℃;
|
82% |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 20 ℃;
|
82% |
|
With N-Bromosuccinimide; In dichloromethane; at 20 ℃;
|
82% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃;
|
80% |
|
With N-Bromosuccinimide; sodium hydride; In hexane; N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h;
|
80% |
|
With N-Bromosuccinimide; In tetrahydrofuran; for 4h; Inert atmosphere; Cooling with ice;
|
80% |
|
With N-Bromosuccinimide; silica gel; In 1,2-dichloro-ethane; at 20 ℃; for 18h; Inert atmosphere; Darkness;
|
78% |
|
With N-Bromosuccinimide; In tert-butyl methyl ether; at 100 ℃; for 12h; Sealed tube;
|
78% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane;
|
76% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 24h; Darkness;
|
75% |
|
With N-Bromosuccinimide; In tetrahydrofuran; at 40 ℃; for 20h;
|
75% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 22 ℃; for 16h; Inert atmosphere; Darkness;
|
74% |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In dichloromethane; at 20 ℃;
|
73% |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In dichloromethane; at 20 ℃;
|
73% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h;
|
72% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 24h; Darkness;
|
72% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 0.5h; Cooling with ice;
|
68% |
|
With hydrogen bromide; dimethyl sulfoxide; at 80 ℃; for 10h;
|
67% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 6h;
|
66% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 2h; Cooling with ice;
|
65% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 12h; Darkness;
|
64% |
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃; for 12h;
|
63% |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; for 3h; Inert atmosphere;
|
61.5% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 4h;
|
59% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20 ℃; for 2h; Inert atmosphere;
|
55% |
|
With bromine; acetic acid; In ethanol;
|
52.1% |
|
With bromine; In carbon disulfide; at 50 ℃; for 5h;
|
47% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; Cooling with ice;
|
47% |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; toluene; at 0 - 20 ℃; for 1.5h; Inert atmosphere; Schlenk technique;
|
43% |
|
With N-Bromosuccinimide; In tetrahydrofuran; at 20 ℃; for 3.66667h;
|
43.8% |
|
With potassium bromate; hydrogenchloride; acetic acid; potassium bromide;
|
|
|
With carbon disulfide; bromine;
|
|
|
With bromine; acetic anhydride; acetic acid;
|
|
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 18 ℃; for 4.5h; Yield given;
|
|
|
With tetra-N-butylammonium tribromide; In chloroform; for 0.5h; Ambient temperature;
|
100 % Chromat. |
|
With N-Bromosuccinimide; In acetonitrile; at 20 ℃; Inert atmosphere; Cooling with ice;
|
|
|
With N-Bromosuccinimide; In dichloromethane;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide;
|
|
|
With bromine; sodium acetate; acetic acid; at 0 ℃;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃;
|
|
|
With N-Bromosuccinimide; silica gel; potassium carbonate; In dichloromethane; for 24h; Inert atmosphere;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; toluene; at 0 ℃; for 2h; Inert atmosphere;
|
|
|
With N-Bromosuccinimide;
|
|
|
With N-Bromosuccinimide; In tetrachloromethane; for 1h; Reflux;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; toluene; at 0 ℃;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; for 0.5h;
|
|
|
With N-Bromosuccinimide; Inert atmosphere;
|
|
|
With N-Bromosuccinimide; silica gel; In dichloromethane; at 20 ℃;
|
|
|
With N-Bromosuccinimide; silica gel;
|
|
|
With N-Bromosuccinimide; In chloroform; at 20 ℃;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; toluene;
|
|
|
With bromine; In carbon disulfide;
|
|
|
With bromine; acetic anhydride; acetic acid;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 95 ℃; for 12h; Schlenk technique; Inert atmosphere;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide;
|
|
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 20 ℃; for 1h;
|
|
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 25 ℃; for 25h;
|
3.6 g |
|
With N-Bromosuccinimide; In dichloromethane; N,N-dimethyl-formamide; at 20 ℃; for 24h;
|
3.6 g |
|
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 ℃; for 3h;
|
|
|
With N-Bromosuccinimide; In tetrahydrofuran; N,N-dimethyl-formamide; at 40 ℃;
|
|
|
With N-Bromosuccinimide; In toluene;
|
N-nitroso-3,6-dibromocarbazole
acetone
9-benzoyl-3,6-dibromo-carbazole
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione
3,6-dibromo-9-ethyl-9H-carbazole
9-acetyl-3,6-dibromocarbazole
3,6-dibromo-9-methyl-9H-carbazole
3,6-dibromo-1-nitrocarbazole
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97