Your Location:Home >Products >OLED intermediates >Carbazoles >136630-39-2

Product Details
|
Application |
One of the most popular products made available from 2,7-dibromocarbazole is PCDTBT, a well-known polymer semiconductor used for polymer solar cells. A good example for adjusting the solubility of the carbazole compounds by using 2,7-dibromocarbazole as the starting materials is the synthesis of 2,7-dibromo-9-heptadecanylcarbazole. |
|
Description |
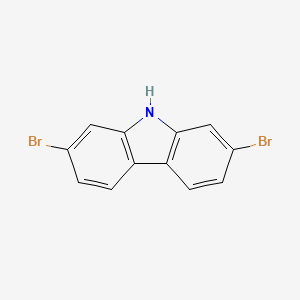
2,7-Dibromocarbazole (2,7-DBCZ) is one of the most frequently detected polyhalogenated carbazoles (PHCZs) in the environmental media. 2,7-dibromocarbazole, also known as 2,7-dibromo-9H-carbazole, is a building block for the synthesis of small molecules of carbazole series or carbazole back-boned polymers in the application of OFETs, OLEDs, PLEDs and OPVs. |
|
Chemical Properties |
White to light yellow powder or crystals |
| XLogP3-AA | 4.8 |
Isomeric SMILES: C1=CC2=C(C=C1Br)NC3=C2C=CC(=C3)Br
InChIKey: QPTWWBLGJZWRAV-UHFFFAOYSA-N
InChI:InChI=1/C12H7Br2N/c13-7-1-3-9-10-4-2-8(14)6-12(10)15-11(9)5-7/h1-6,15H
Polyhalogenated carbazoles (PHCZs), which have the similar structure of dioxin, have been reported ubiquitous in the environments and drawn wide concerns. However, their potential ecological and health risks are still poorly understood. Here, wildtype zebrafish embryos were used to evaluate the environmental risks of 2,7-dibromocarbazole (2,7-DBCZ), 3,6-dibromocarbazole (3,6-DBCZ), and 3,6-dichlorocarbazole (3,6-DCCZ). 2,7-DBCZ was the most toxic compound with the 96-h LC50 value of 581.8 ± 29.3 μg·L−1 and the EC50 value of 201.5 ± 6.5 μg·L−1 for pericardial edema.
It were used to evaluate the environmental risks of 2,7-dibromocarbazole (2,7-DBCZ), 3,6-dibromocarbazole (3,6-DBCZ), and 3,6-dichlorocarbazole (3,6-DCCZ). 2,7-DBCZ was the most …
Here, we investigated the tissue-specific accumulation, depuration, and histopathological effects of two typical PHCZs, 3,6-dichlorocarbazole (36-CCZ) and 2,7-dibromocarbazole (27-BCZ), in adult zebrafish at three levels (0, 0.15 μg/L (5 × environmentally relevant level), and 50 μg/L (1/10 LC50). The lowest concentrations of 36-CCZ (1.2 μg/g ww) and 27-BCZ (1.4 μg/g ww) were observed in muscle, and the greatest concentrations of 36-CCZ (3.6 μg/g ww) and 27-BCZ (4 μg/g ww) were detected in intestine among the tested tissues.
4,4′-dibromo-[1,1′-biphenyl]-2,2′-diamine
{4-[7-bromo-9-(4-trimethylsilanylethynylphenyl)-9H-carbazol-2-ylethynyl]phenyl}dimethylamine
4,4'-dibromo-2-nitro-biphenyl
4,4'-dibromo-2,2'-dinitrobiphenyl
2,7-dicyanocarbazole
2,7-dibromo-9-(4-nitrophenyl)-9H-carbazole
2,7-dibromo-9-(2-ethyl-hexyl)-9H-carbazole
2,7-dibromo-9-octyl-9H-carbazole
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97
CAS:333432-28-3
Molecular Formula:C15H15BO2
Molecular Weight:238.09