Your Location:Home >Products >OLED intermediates >Thiophenes >16839-97-7
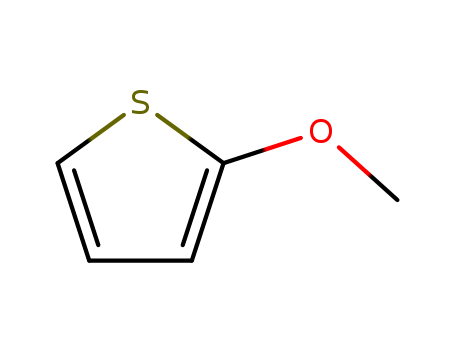

Product Details
Chemical Properties
CLEAR YELLOW LIQUID
Uses
2-Methoxythiophene was used in thermal reaction (60°C) of (C5Me5)Rh(PMe3)(Ph)H.
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 3697, 1953 DOI: 10.1021/ja01111a027
General Description
2-Methoxythiophene is a heterocyclic methyl enol ether and its reaction with o-quinone monoimide was studied. The intramolecular and intermolecular geometries of crystals of 2-methoxythiophene were investigated. Kinetics of the hydronium-ion catalysed hydrolysis of 2-methoxythiophene was reported.
InChI:InChI=1/C5H6OS/c1-6-5-3-2-4-7-5/h2-4H,1H3
A ligand-free, powerful, and practical method for mono and polymethoxylation of unactivated aryl bromides has been developed; CuCl was used as catalyst, HCOOMe as cocatalyst, and methanolic MeONa as both nucleophile and solvent. This eco-friendly procedure is characterized by operational simplicity, inexpensive substrates (unactivated mono to polybromoarenes), full conversion, and direct recovery of pure MeOH.
The invention provides compounds of formula la, lb and Ic: [Formula Ia, Ib, and Ic] and salts thereof, wherein variables are as described in the specification, as well as compositions comprising a compound of formula Ia-Ic, methods of making such compounds, and methods of using such compounds, e.g., as inhibitors of bacterial RNA polymerase and as antibacterial agents.
Method for preparing compounds of the formula (III) by reacting compounds of the formula (II) with a) an alcoholate or b) an alcohol R1-OH and a base in the presence of a Cu-containing catalyst and of a ligand, where X1-5 are independently of one another either carbon or nitrogen, or in each case two adjacent X1R1, with i=1?6, linked by a formal double bond together O, S, NRH or Nrl. The ligands preferably employed are acyclic and/or cyclic oligo- and polyglycols, oligo- and polyamides or oligo- and polyamine glycols of the general formula (IV) k is an integer >0 and n is an integer >1; X and Y are independently of one another O, NH or NR1.
The preparation of thiophene ethers generally requires forcing conditions thus limiting the choice of alkyl substituent. Herein, we report the first successful generally applicable conditions for the selective O-alkylation of 2(5H)-thiophenone.
methanol

diiodo-2,4 thiophene

3-thienyl iodide

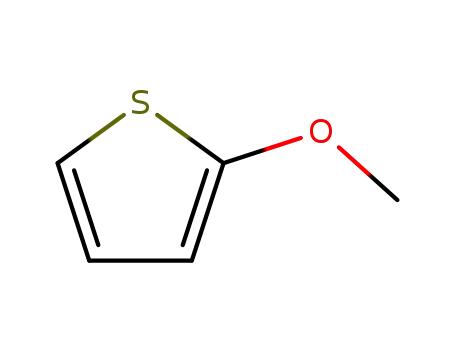
2-methoxythiophene

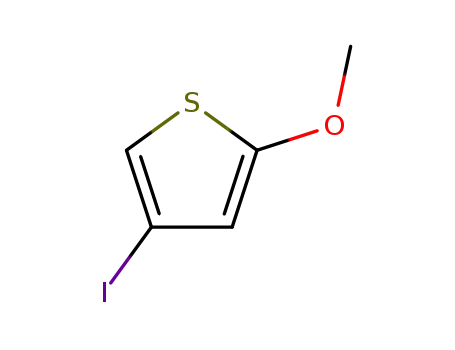
4-Iodo-2-methoxythiophene

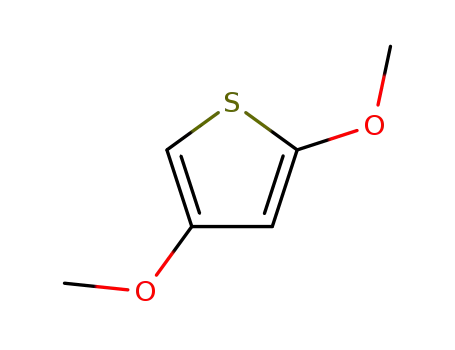
2,4-dimethoxythiophene
| Conditions | Yield |
|---|---|
|
With
sodium;
for 3h;
Title compound not separated from byproducts;
Heating;
|
66 % Chromat. 5 % Chromat. 15 % Chromat. 13 % Chromat. |
|
With
sodium;
for 3h;
Heating;
|
5 % Chromat. 15 % Chromat. 13 % Chromat. 66 % Chromat. |
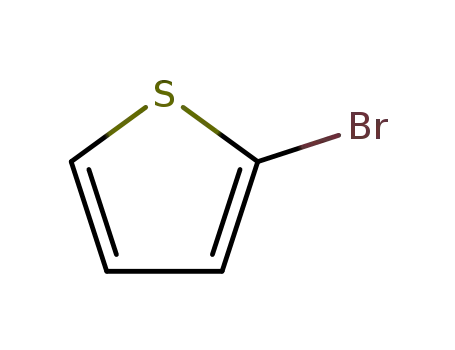
2-bromothiophene

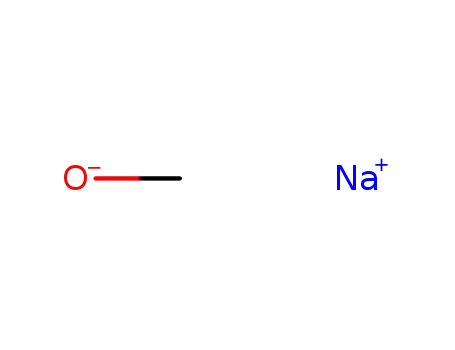
sodium methylate


2-methoxythiophene
| Conditions | Yield |
|---|---|
|
polyethylene glycol dimethyl ether; copper(I) bromide;
In
methanol;
at 90 ℃;
for 8h;
|
86.4% |
|
With
copper(I) bromide;
In
methanol;
for 5h;
Heating;
|
83% |
|
copper(I) bromide;
In
methanol;
for 6h;
Heating;
|
83% |
|
With
methanol; Methyl formate; copper(l) chloride;
at 115 ℃;
for 2h;
Autoclave;
Green chemistry;
|
80% |
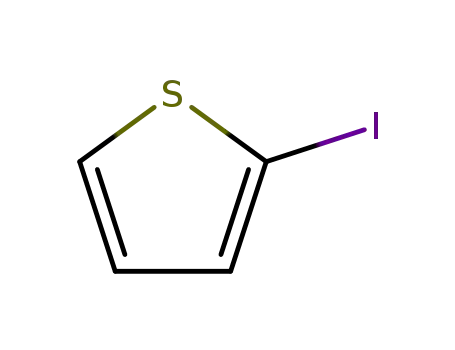
2-Iodothiophene

sodium methylate

2-bromothiophene
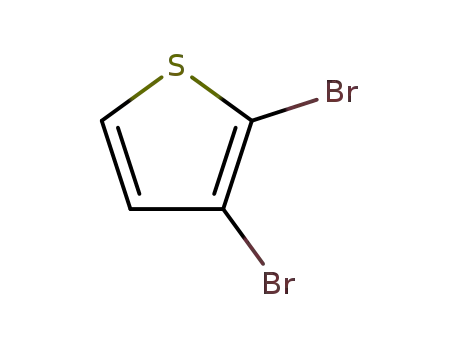
2,3-dibromothiophen
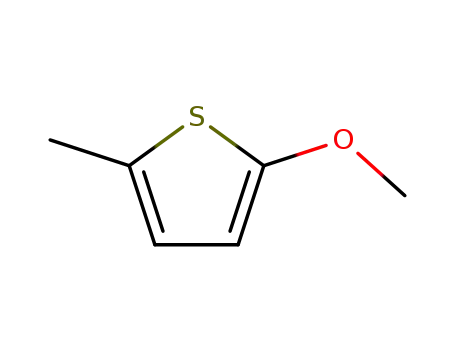
thiophene, 2-methoxy-5-methyl-
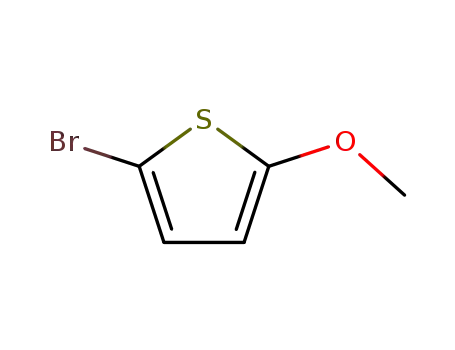
5-bromo-2-methoxythiophene
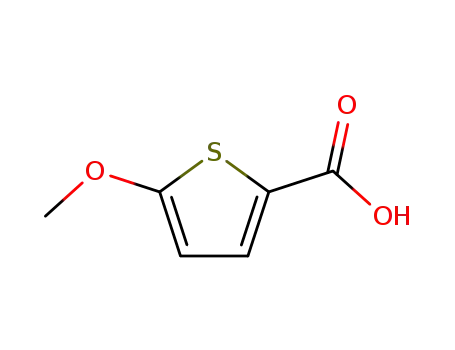
5-methoxythiophene-2-carboxylic acid
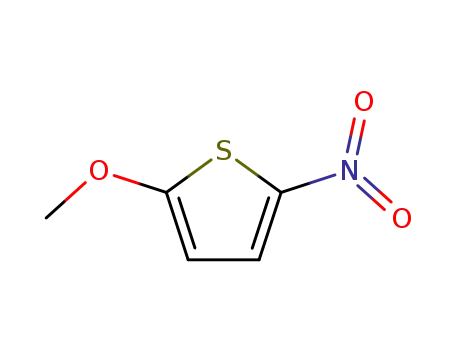
2-methoxy-5-nitrothiophene
CAS:419536-33-7
Molecular Formula:C18H14BNO2
Molecular Weight:287.1
CAS:14282-76-9
CAS:17573-92-1