Your Location:Home >Products >Organic phosphines >Other organic phosphines >4731-53-7


Product Details
Uses
Tri-n-octylphosphine is used for coating zinc sulfide shells on cadmium-selenium quantum dot core by successive ionic layer adsorption and reaction method. It acts as a precursor to trioctylphosphine oxide. Further, it is used as a solvent for cadmium and selenium precursors. It also serves as a common reagent in the chemical synthesis of nanoparticles. It acts as a precursor to trioctylphosphine oxide.
InChI:InChI=1/C24H51P/c1-4-7-10-13-16-19-22-25(23-20-17-14-11-8-5-2)24-21-18-15-12-9-6-3/h4-24H2,1-3H3
On the basis of evidence from 31P NMR spectroscopy, and using PbSe as a model, we propose two simultaneous mechanisms through which monomers are formed in preparations of lead chalcogenide nanocrystals (NCs). In one mechanism, selenium is delivered as a Se2- species, whereas in the other, Se0 reacts with metal already reduced by the organophosphine. This latter mechanism helps explain the sensitivity of NC preparations to the purity of organophosphines and allows the rational modification of batch NC reactions to increase yield. Copyright
A rapid method for the reduction of secondary phosphine oxides under mild conditions has been developed, allowing simple isolation of the corresponding free phosphines. The methodology involves the use of pinacol borane (HBpin) to effect the reduction while circumventing the formation of a phosphine borane adduct, as is usually the case with various other commonly used borane reducing agents such as borane tetrahydrofuran complex (BH3?THF) and borane dimethyl sulfide complex (BH3?SMe2). In addition, this methodology requires only a small excess of reducing agent and therefore compares favourably not just with other borane reductants that do not require a metal co-catalyst, but also with silane and aluminium based reagents. (Figure presented.).
A method for increasing the rate of phosphine oxide reduction, preferably during a Wittig reaction comprising use of an acid additive is provided. A room temperature catalytic Wittig reaction (CWR) the rate of reduction of the phosphine oxide is increased due to the addition of the acid additive is described. Furthermore, the extension of the CWR to semi-stabilized and non-stabilized ylides has been accomplished by utilization of a masked base and/or ylide-tuning.
One ring no longer rules them all: Employment of 2.5-10 mol % of 4-nitrobenzoic acid with phenylsilane led to the development of a room temperature catalytic Wittig reaction (see scheme). Moreover, these enhanced reduction conditions also facilitated the use of acyclic phosphine oxides as catalysts for the first time. A series of alkenes were produced in moderate to high yield and selectivity. Copyright
An efficient method for the reduction of phosphine oxide derivatives into their corresponding phosphines is described. The system InBr3/TMDS allows the reduction of different secondary and tertiary phosphine oxides as well as aliphatic and aromatic phosphine oxides.
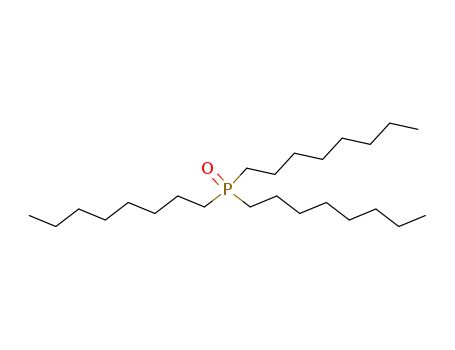
cyanex-921


TOP
| Conditions | Yield |
|---|---|
|
With
pinacolboronic acid;
In
acetonitrile;
at 100 ℃;
for 120h;
Inert atmosphere;
Glovebox;
Sealed tube;
Schlenk technique;
|
57% |
|
With
1,1,3,3-Tetramethyldisiloxane;
titanium(IV) isopropylate;
In
various solvent(s);
at 100 ℃;
for 10h;
|
|
|
With
indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane;
In
toluene;
at 100 ℃;
for 18h;
Inert atmosphere;
Sealed tube;
|
|
|
With
copper(II) trifluoromethanesulfonate; 1,1,3,3-Tetramethyldisiloxane;
In
toluene;
at 100 ℃;
Inert atmosphere;
|
70 %Spectr. |
|
With
phenylsilane; N-ethyl-N,N-diisopropylamine; 4-nitro-benzoic acid;
In
toluene;
at 100 ℃;
for 0.166667h;
Inert atmosphere;
|
73 %Spectr. |
|
With
phenylsilane; N-ethyl-N,N-diisopropylamine; 4-nitro-benzoic acid;
In
toluene;
at 100 ℃;
for 0.166667h;
Reagent/catalyst;
Sealed tube;
Inert atmosphere;
Schlenk technique;
|

di-n-octylmagnesium


TOP
| Conditions | Yield |
|---|---|
|
With
phosphorus trichloride;
iron(III)-acetylacetonate;
In
tetrahydrofuran; n-heptane;
for 0.5h;
Heating;
|
85% |
|
With
phosphorus trichloride;
|
84% |
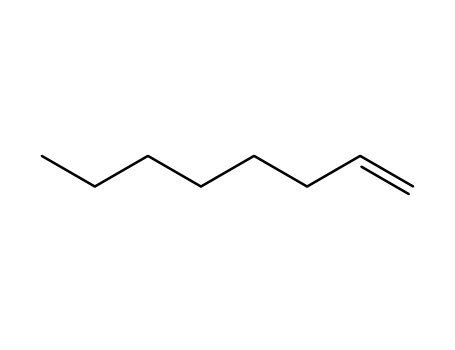
oct-1-ene
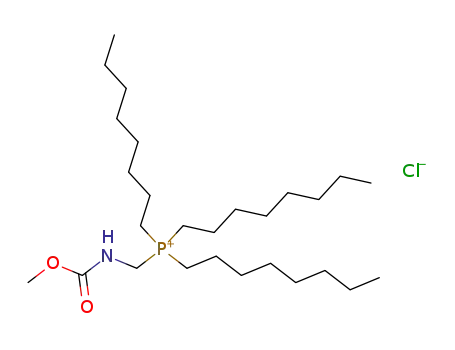
(Methoxycarbonylamino-methyl)-trioctyl-phosphonium; chloride
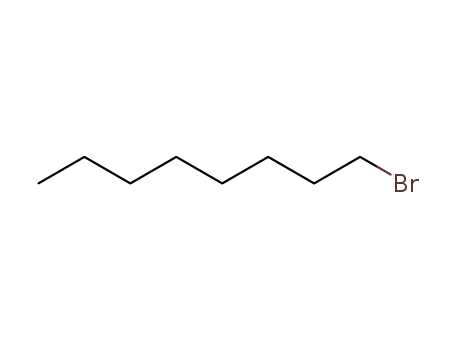
1-bromo-octane

di-n-octylmagnesium

cyanex-921

tri-n-octylphosphine sulfide

trioctylphosphane selenide
dioctylphosphinic acid
CAS:92343-46-9
Molecular Formula:C<sub>8</sub>H<sub>10</sub>O<sub>3</sub>
Molecular Weight:154.16
CAS:1189047-28-6
CAS:20445-88-9