Your Location:Home >Products >OLED intermediates >Fluorenes >19261-06-4
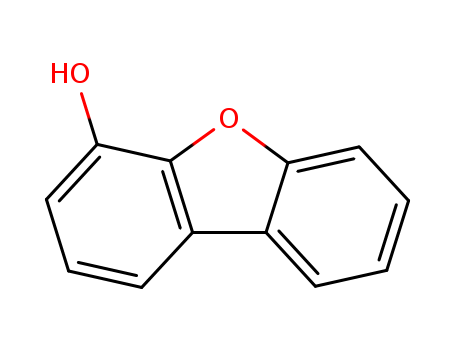

Product Details
InChI:InChI=1/C12H8O2/c13-10-6-3-5-9-8-4-1-2-7-11(8)14-12(9)10/h1-7,13H
Following the discovery of dibenzo[b,d]thiophen-4-yl)-2-morpholino-4H- chromen-4-one (NU7441) (Leahy, J. J. J.; Golding, B. T.; Griffin, R. J.; Hardcastle, I. R.; Richardson, C.; Rigoreau, L.; Smith, G. C. M. Bioorg. Med. Chem. Lett. 2004, 14, 6083-6087) as a potent inhibitor (IC50 = 30 nM) of DNA-dependent protein kinase (DNA-PK), we have investigated analogues in which the chromen-4-one core template has been replaced by aza-heterocyclic systems: 9-substituted 2-morpholin-4-ylpyrido[1,2-a]pyrimidin-4-ones and 8-substituted 2-morpholin-4-yl-1H-quinolin-4-ones. The 8- and 9-substituents were either dibenzothiophen-4-yl or dibenzofuran-4-yl, which were each further substituted at the 1-position with water-solubilizing groups [NHCO(CH 2)nNR1R2, where n = 1 or 2 and the moiety R1R2N was derived from a library of primary and secondary amines (e.g., morpholine)]. The inhibitors were synthesized by employing a multiple-parallel approach in which the two heterocyclic components were assembled by Suzuki-Miyaura cross-coupling. Potent DNA-PK inhibitory activity was generally observed across the compound series, with structure activity studies indicating that optimal potency resided in pyridopyrimidin-4- ones bearing a substituted dibenzothiophen-4-yl group. Several of the newly synthesized compounds (e.g., 2-morpholin-4-yl-N-[4-(2-morpholin-4-yl-4-oxo-4H- pyrido[1,2-a]pyrimidin-9-yl)dibenzothiophen-1-yl]acetamide) combined high potency against the target enzyme (DNA-PK IC50 = 8 nM) with promising activity as potentiators of ionizing radiation-induced cytotoxicity in vitro.
(Graph Presented) The design of novel, functionalized semiquinone (SQ) ligands which combine structural rigidity and electron-withdrawing, electron-donating, and electroneutral substituents enables investigation of multiple structure-property relationships and building blocks for new materials, including components of sensors, switches, and molecular spintronics. Along these lines, we report the synthesis of several new SQ ligands containing fused heterocyclic ring systems. Using both electron paramagnetic resonance spectroscopy and quantum chemical calculations, we show how spin density is affected by the fused ring system substituents.
A new simple protocol for the conversion of arylboronic acids to hydroxyarenes was achieved using molecular oxygen in the presence of tetrabutylammonium borohydride under blacklight irradiation (360 nm). A radical chain mechanism in which a superoxide ion (O2?.) plays a key role is proposed.
Cellulose catalyzed oxidative hydroxylation of aryl and hetero-arylboronic acids to the corresponding phenols under metal and base free strategy has been demonstrated. The sustainable ipso-hydroxylation takes place using hydrogen peroxide as an oxidant in water under mild condition in shorter period of time. Interestingly, easy recovery and reusability of heterogeneous catalyst without significant loss in catalytic yield makes the protocol environmentally benign.
A novel and efficient strategy for the ipso-hydroxylation of arylboronic acids to phenols has been developed using inexpensive, readily available, air-stable water-soluble povidone iodine as catalyst and aqueous hydrogen peroxide as oxidizing agent. The reactions were performed at room temperature under metal-, ligand- and base-free condition in a short reaction time. The corresponding substituted phenols were obtained in moderate to good yields by oxidative hydroxylation of arylboronic acids in aqueous medium.
A metal-free and base-free Cl3CCN mediated method was developed for the ipso-hydroxylation of aryl boronic acids to their corresponding phenols, which was promoted by a key unstable Lewis adduct intermediate. This transformation has broad functional group tolerance, and late-stage functionalization was successful as well. After simple investigation, two pathways (radical/ionic mechanism) were suggested, and the beneficial action of blue light needs to be further studied.
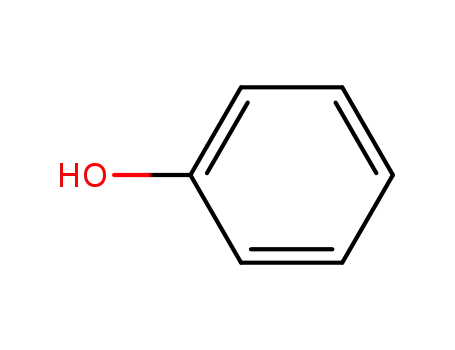
phenol

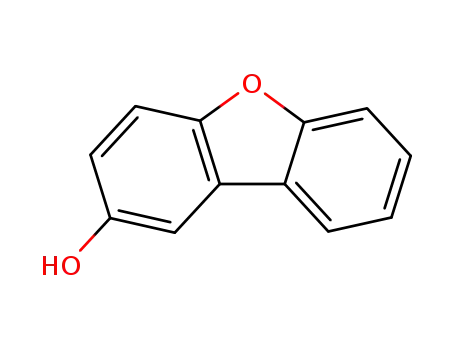
2-hydroxydibenzofuran

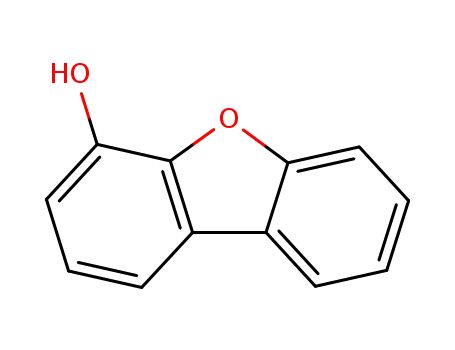
4-hydroxydibenzofuran

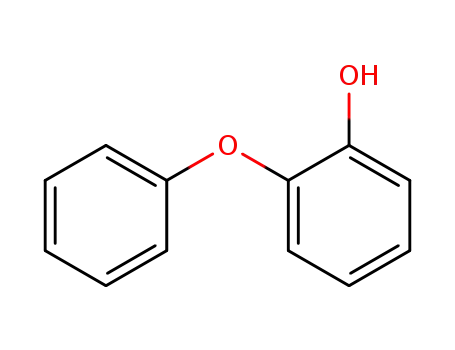
o-Phenoxyphenol

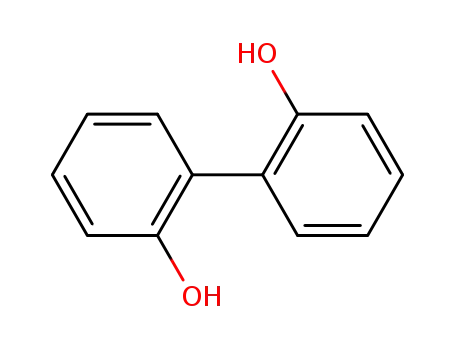
2,2'-dihydroxybiphenyl
| Conditions | Yield |
|---|---|
|
With
oxygen;
In
benzene;
at 499.84 ℃;
for 0.0125h;
Further Variations:;
Reagents;
Temperatures;
Product distribution;
|
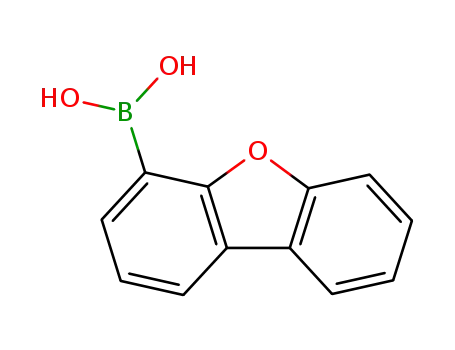
4-dibenzofurylboronic acid


4-hydroxydibenzofuran
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
In
water;
at 20 ℃;
for 0.0833333h;
|
95% |
|
With
sodium hydroxide; dihydrogen peroxide;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
|
94% |
|
With
copper(II) ferrite; water; sodium hydroxide;
at 40 ℃;
for 24h;
Green chemistry;
|
93% |
|
With
dihydrogen peroxide;
In
water;
at 30 ℃;
Green chemistry;
|
93% |
|
With
carbonodihydrazide; caesium carbonate;
In
water;
at 80 ℃;
for 12h;
|
90% |
|
With
Fe2O3-SiO2 nanoparticles; air;
In
water;
at 50 ℃;
for 3h;
Green chemistry;
|
90% |
|
With
water; caesium carbonate; hydrazine hydrate;
at 80 ℃;
for 12h;
|
83% |
|
With
tert.-butylhydroperoxide; oxygen; trichloroacetonitrile;
In
decane; acetonitrile;
at 20 ℃;
for 12h;
Irradiation;
|
82% |
|
With
rose bengal; triethylamine;
In
ethanol;
at 25 ℃;
for 12h;
Schlenk technique;
Irradiation;
|
61% |
|
With
dihydrogen peroxide;
In
ethanol; water;
at 60 ℃;
for 2h;
|
57.6% |
|
With
oxygen; tetrabutylammonium borohydride;
In
acetonitrile;
at 20 ℃;
for 4h;
Irradiation;
|
49% |
|
With
water; dihydrogen peroxide;
In
diethyl ether;
at 0 ℃;
for 1.5h;
Heating / reflux;
|
|
|
With
dihydrogen peroxide; sodium hydroxide;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
Inert atmosphere;
|
|
|
With
urea hydrogen peroxide adduct;
In
acetonitrile;
at 20 ℃;
Cooling with ice;
Inert atmosphere;
|
60.6 g |
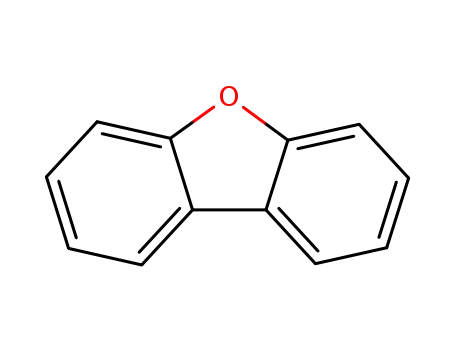
dibenzofuran
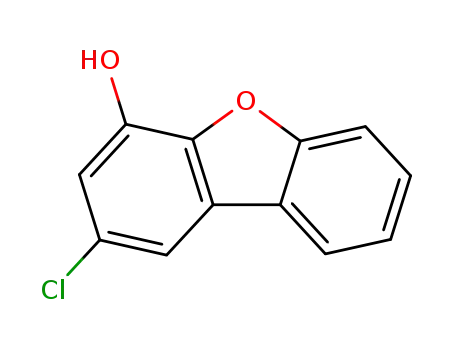
2-chloro-dibenzofuran-4-ol
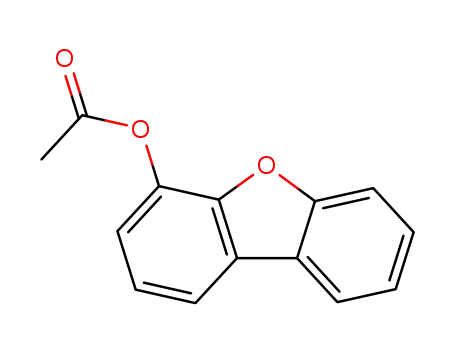
4-acetoxydibenzofuran
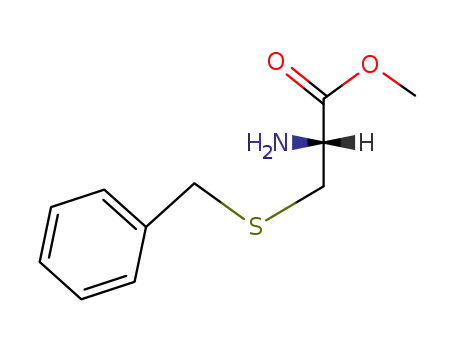
S-benzylcysteine methyl ester
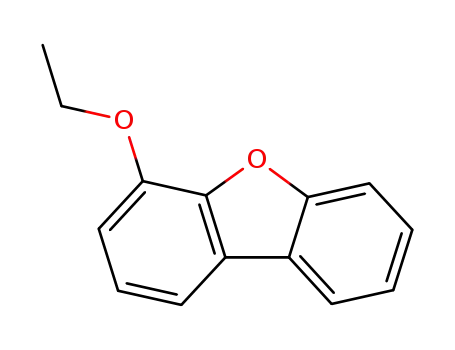
4-ethoxy-dibenzofuran
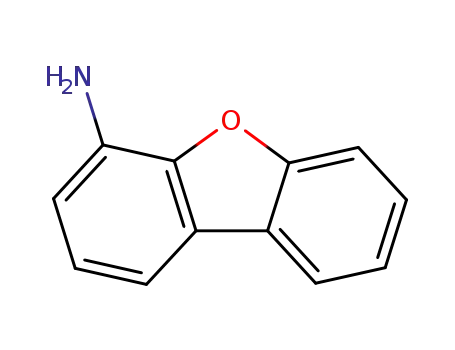
4-aminodibenzofuran
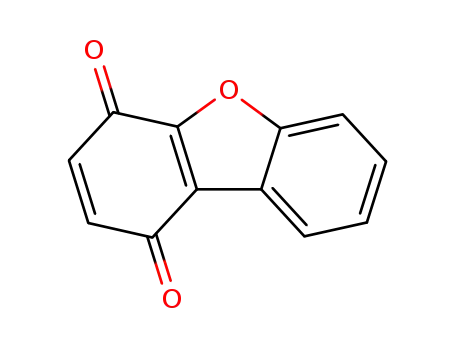
Dibenzofuran-1,4-chinon
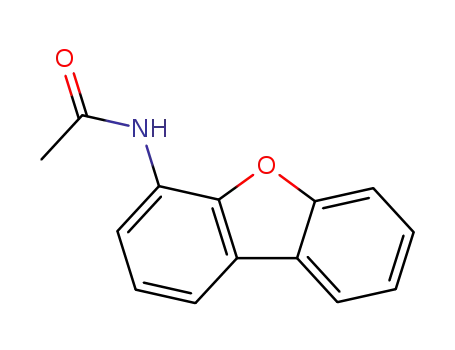
N-(4-dibenzofuranyl)acetamide
CAS:1081-34-1
CAS:201138-91-2