Your Location:Home >Products >OLED intermediates >Fluorenes >781-73-7
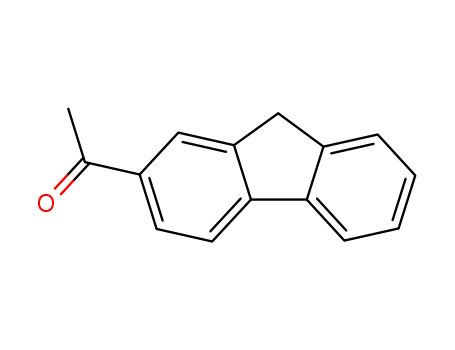

Product Details
Description
2-Acetylfluorene is a crystalline powder of light yellow and is stable under normal temperatures and pressures. 2-Acetylfluorene is incompatible with strong oxidising agents. 2-Acetylfluorene on decomposition releases carbon monoxide and carbon dioxide.
Chemical Properties
2-Acetylfl uorene is a light yellow crystalline powder and is stable under normal temperatures and pressures. 2-Acetylfl uorene is incompatible with strong oxidizing agents. On decomposition, it releases carbon monoxide and carbon dioxide.
Health Hazard
Exposures to 2-acetylfl uorene cause irritations to the eyes, skin, respiratory tract, and digestive tract. The toxicological properties have not been fully investigated.
storage
2-Acetylfl uorene should be stored in a cool, dry place, in a tightly sealed container.
Purification Methods
Crystallise acetylfluorene from EtOH (solubility is 60g/800mL) or Me2CO (solubility is 60g/400mL). The oxime [110827-07-1] has m 192-193.5o and the 2,4-dinitrophenylhydrazone [109682-26-0] has m 261-262o. [Ray & Rieveschl Org Synth Coll Vol III 23 1973.]
Precautions
Occupational workers should avoid exposures to 2-acetylfl uorene and breathing dust, vapor, mist, or gas. They should avoid contact with the skin and eyes, and avoid ingestion and inhalation. Workers should wear appropriate protective gloves to prevent skin exposure and chemical-splash goggles.
InChI:InChI=1/C15H12O/c1-10(16)11-6-7-15-13(8-11)9-12-4-2-3-5-14(12)15/h2-8H,9H2,1H3
New platforms for trinuclear complexes, 1,3,5-tris(fluoren-2-yl-R)benzene (Ph{2-FluRH}3; R = H (2a), 6-tBu (2b), 7-tBu (2c), Tet (2d) (Tet = 2,2,5,5-tetramethyl-tetrahydrobenzofluorene), were synthesized via an acid-catalyzed cyclotrimerization of the corresponding substituted 2-acetylfluorenes. Subsequent nucleophilic addition of the [Ph{2-FluR}3]3? trianions onto 6,6,-dimethylfulvene afforded the corresponding isopropylidene-bridged pro-ligands Ph{Me2C(2-FluRH)(C5H5)}3 (3a-d). Discrete trinuclear tris(dichloro-ansa-zirconocene and hafnocene), Ph[{Me2C(2-FluR)(C5H4)}MX2]3 (X = Cl: 4b-d-Zr, 4c,d-Hf) were prepared by salt metathesis reactions. Some zirconium complexes were further alkylated towards the corresponding tris(dialkyls) (X = Me: 5c,d-Zr; X = CH2SiMe3: 6c-Zr). The structures of these metal complexes were determined by elemental analyses, and by 1D, inverse 2D heteronuclear correlation, and DOSY NMR spectroscopy, as well as by theoretical computations. Those studies revealed the existence of two isomers, of C3 and C1 symmetry respectively, originating from the mutual orientation of the three ansa-metallocene fragments. Preliminary studies on the catalytic performances of the dichloro complexes, upon activation with MAO, in ethylene and propylene homopolymerization and ethylene/1-hexene copolymerization were carried out and compared to those of the monometallic analogues under identical conditions.
The synthesis of fluorenes by intramolecular Pd-catalyzed C(sp 2)-H activation of 2-arylbenzyl chlorides was conducted at room temperature by using commercially available triphenylphosphine and pivalic acid as ligands. The desired reactions proceeded efficiently at room temperature, and various substrates were converted into the corresponding fluorene derivatives in excellent yields.
Enzymes with a hydrophobic binding site and an active site lysine have been suggested to be promiscuous in their catalytic activity. β-Lactoglobulin (BLG), the principle whey protein found in milk, possesses a central calyx that binds non-polar molecules. Here, we report that BLG can catalyze the retro-aldol cleavage of α,β-unsaturated aldehydes making it a naturally occurring protein capable of catalyzing retro-aldol reactions on hydrophobic substrates. Retroaldolase activity was seen to be most effective on substrates with phenyl or naphthyl side-chains. Use of a brominated substrate analogue inhibitor increases the product yield by a factor of three. BLG's catalytic activity and its ready availability make it a prime candidate for the development of commercial biocatalysts.
Converting organoboron compounds into the corresponding radicals has broad synthetic applications in organic chemistry. To achieve these transformations, various strong oxidants such as Mn(OAc)3, AgNO3/K2S2O8, and Cu(OAc)2, in stoichiometric amounts are required, proceeding by a single-electron transfer mechanism. Established herein is a distinct strategy for generating both aryl and alkyl radicals from organotrifluoroborates through an SH2 process. This strategy is enabled by using water as the solvent, visible light as the energy input, and diacetyl as the promoter in the absence of any metal catalyst or redox reagent, thereby eliminating metal waste. To demonstrate its synthetic utility, an efficient acetylation to prepare valuable aryl (alkyl) methyl ketones is described and applications to construct C?C, C?I, C?Br, and C?S bonds are also feasible. Experimental evidence suggests that triplet diacetyl serves as the key intermediate in this process.
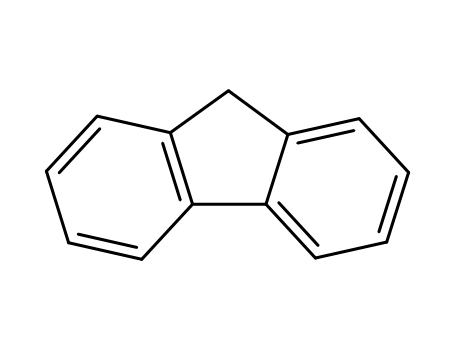
9H-fluorene

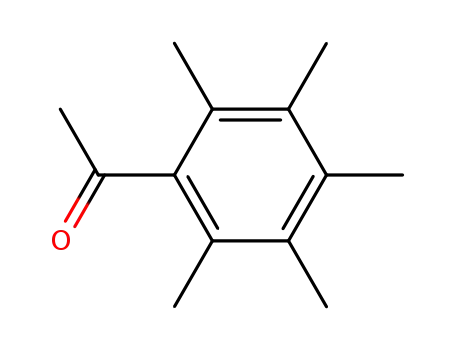
2',3',4',5',6'-pentamethylacetophenone

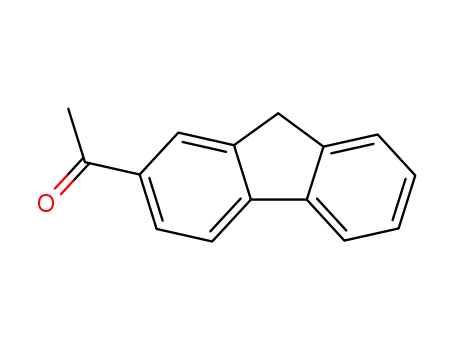
1-(9H-fluoren-2-yl)ethanone

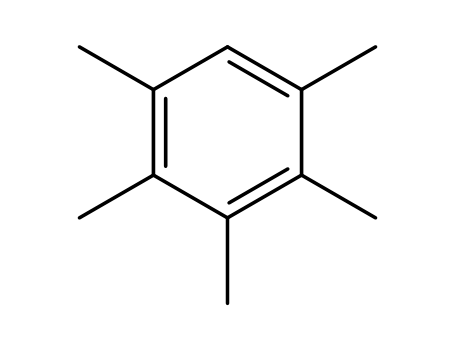
pentamethylbenzene,
| Conditions | Yield |
|---|---|
|
trifluoroacetic acid;
for 10h;
Heating;
|
99% 44% |
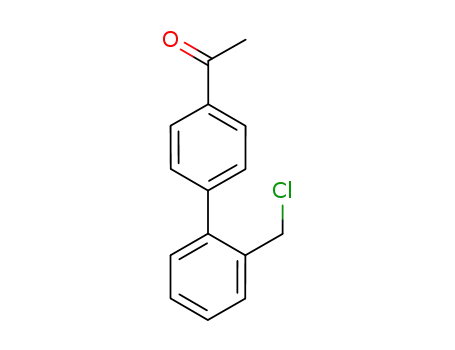
2-(4-acetylphenyl)benzyl chloride


1-(9H-fluoren-2-yl)ethanone
| Conditions | Yield |
|---|---|
|
With
bis-triphenylphosphine-palladium(II) chloride; caesium carbonate; Trimethylacetic acid;
In
tetrahydrofuran;
at 25 ℃;
for 18h;
Glovebox;
Schlenk technique;
Inert atmosphere;
|
94% |
|
With
palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl;
In
1,2-dimethoxyethane;
at 100 ℃;
for 12h;
Inert atmosphere;
|
48% |

9H-fluorene
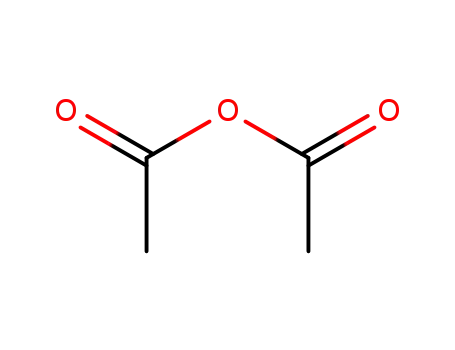
acetic anhydride
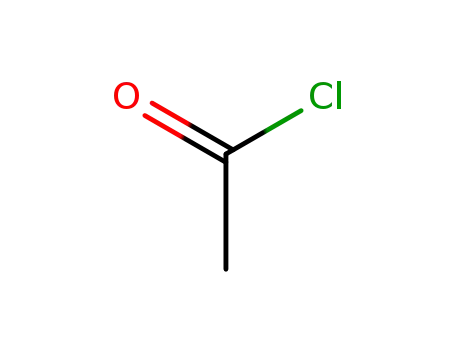
acetyl chloride
N-Acetylimidazole
2-ethylfluorene
2-acetylfluoren-9-one
fluorenone-2-carboxylic acid
1-(9H-fluoren-2-yl)ethan-1-ol
CAS:13029-09-9
CAS:131409-18-2
CAS:1689-64-1