Your Location:Home >Products >OLED intermediates >Fluorenes >1689-64-1
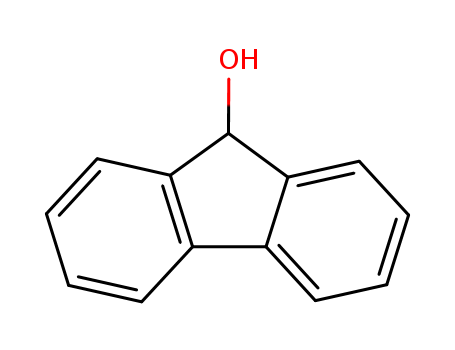

Product Details
Chemical Properties
white powder
Uses
9-Fluorenol is used in Organic Synthesis, Pharmaceuticals, Agrochemicals and Dyestuffs.
Application
9-Fluorenol is a wake-promoting agent and is considered a next-generation anti-drowsiness drug. It is also a potential environmental carcinogen.9-Fluorenol is a dopamine reuptake inhibitor with IC50 of 9 μM, and a major metabolite of a compound developed as a wakefulness-promoting agent.
Preparation
Synthesis of 9-Fluorenol: A 5-g sample of 9-fluorenone is dissolved in 30 mL of warm ethanol in a 100-mL beaker. To this solution is added, dropwise, 10 mL of a reducing reagent, freshly prepared, which consists of 200 mg sodium methoxide,10 mL methanol, and 0.4 g sodium borohydride.This solution is allowed to stand undisturbed for 10-20 min. A color change is observed as the reaction proceeds from the yellow 9-fluorenone to the white 9-fluorenol.The product is precipitated by the addition of 50 mL of water and neutralized with 0.1 M HCl, vacuum filtered, and washed with cold water to remove any residual inorganic salt formed by the excess of the sodium borohydride reagent and hydrochloric acid.The dried crude product is sufficiently pure for characterization by melting point, infrared spectra,and NMR.The crude product melting point is 153°C, with a product yield of 95-100%.A Synthesis of 9-Fluorenol: Sodium Borohydride Reduction of 9-Fluorenone
Definition
ChEBI: 9-Fluorenol is a member of the class of hydroxyfluorenes that is 9H-fluorene substituted by a hydroxy group at position 9 (the non-aromatic carbon). It has a role as an animal metabolite. It is a member of hydroxyfluorenes and a secondary alcohol.
Synthesis Reference(s)
The Journal of Organic Chemistry, 57, p. 6313, 1992 DOI: 10.1021/jo00049a045Tetrahedron Letters, 13, p. 343, 1972
Synthesis
9-Fluorenol is prepared by reacting 9-fluorenone with THF solution of phosphazene under the action of boron catalyst.Add 9-fluorenone (0.8 mmol), a 0.2 M THF solution of phosphazene (80 μL, 0.016 mmol) and a 0.0762 M THF solution of boron catalyst (420 μL, 0.032 mmol) to a scintillation vial with magnetic stir bar in a glove box. Place the scintillation vial in a Parr reactor. Seal the reactor. Pressurize the reactor with hydrogen gas. Heat reaction mixture at 75°C (inside the reactor). Stir the reaction mixture (1000 rpm) for 20 hours at 75°C. Cool the mixture to room temperature and vent the Parr reactor to obtain 9-hydroxyfluoren. Analyze the reaction mixture by 1H NMR spectroscopy using CDCl3 as solvent.Fig the synthetic method of 9-Fluorenol
InChI:InChI=1/C13H10O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8,13-14H
Fluorenyl ester-armed cyclen gave fluorenone and related decomposition compounds upon photoirradiation. The reaction was effectively suppressed by the formation of an octacoordinated Ca2+ complex while Na+ and other alkali/alkaline earth metal cations had little influence. Since the production efficiency of fluorescent fluorenone related well to the concentration of the Ca2+ ion, the photoreaction of this armed cyclen offered the naked-eye detection of Ca2+ ion in aqueous samples. The Royal Society of Chemistry 2005.
We report synthesis and characterization of a mononuclear iron(III) complex [Fe(LH2)Cl2]+, 1 having tridentate ligand LH2 and their application in oxidation of alkanes. Complex, 1 was characterized by various analytical, spectroscopic, electrochemical, crystallographic and theoretical studies. The molecular structure determination showed the presence of distorted square pyramidal FeN3Cl2 coordination sphere. Selective oxidation of benzyl alcohol, fluorene, adamantane, 1-phenyl ethanol and n-octane in presence of catalytic amount [FeIII(LH2)Cl2]+ with H2O2 under mild conditions give products in good to excellent yields through FeIV[dbnd]O intermediate. Several studies were done to support the reactive intermediate. Generation of FeIV[dbnd]O intermediate was also supported by UV–visible titration of [FeIII(LH2)Cl2]+ and H2O2 at low temperature and their visual colour change from red (500 nm, ? = 1050 M? 1 cm? 1) to green (770 nm, ? = 153 M? 1 cm? 1).
Methylytterbium iodide (CH3YbI) has been found to react with active hydrogen compounds such as phenylacetylene and fluorene to give phenylpropynoic acid and fluorenol in modest yields.The reactivity of CH3YbI to these active hydrogen compounds is far higher than of CH3MgI.
An efficient and versatile method was used for the diastereoselective synthesis of achiral and racemic spirohydantoins based on ketones 4, 5, 7 and 10.
-
-
The crystal structures of the haem domains of Ala330Pro and Ile401Pro, two single-site proline variants of CYP102A1 (P450BM3) from Bacillus megaterium, have been solved. In the A330P structure, the active site is constricted by the relocation of the Pro329 side chain into the substrate access channel, providing a basis for the distinctive C-H bond oxidation profiles given by the variant and the enhanced activity with small molecules. I401P, which is exceptionally active towards non-natural substrates, displays a number of structural similarities to substrate-bound forms of the wild-type enzyme, notably an off-axial water ligand, a drop in the proximal loop, and the positioning of two I-helix residues, Gly265 and His266, the reorientation of which prevents the formation of several intrahelical hydrogen bonds. Second-generation I401P variants gave high in vitro oxidation rates with non-natural substrates as varied as fluorene and propane, towards which the wild-type enzyme is essentially inactive. The substrate-free I401P haem domain had a reduction potential slightly more oxidising than the palmitate-bound wild-type haem domain, and a first electron transfer rate that was about 10 % faster. The electronic properties of A330P were, by contrast, similar to those of the substrate-free wild-type enzyme. Protein evolution with proline: The crystal structures of two contrasting single-site proline mutants of CYP102A1 (P450BM3) have been solved. The two mutations combine to give a variant that shows substantially enhanced catalytic activity with small non-natural substrates (see graph).
We disclose a novel Pd-catalyzed assembly of fluoren-9-ones by merging of C-H activation and difluorocarbene transfer. ClCF2COONa served as a difluorocarbene precursor to be harnessed as a carbonyl source in this transformation. The current protocol enables us to afford fluoren-9-ones in high yields with excellent functional group compatibility, which also represents the first example of using difluorocarbene as a coupling partner in transition-metal-catalyzed C-H activation.
-
Sterically well-shielded against unsolicited Michael addition and polymerization reactions, α-metalated α-(1,1,3,3-tetramethyl-indan-2-ylidene)acetonitriles added reversibly to three small aldehydes and two bulky ketones at room temperature. Experimental conditions were determined for transfer of the nucleofugal title carbanion unit between different carbonyl compounds. These readily occurring retro-additions via C-C(O) bond fission may also be used to generate different metal derivatives of the nucleofugal anions as equilibrium components. Fluoride-catalyzed, metal-free desilylation admitted carbonyl addition but blocked the retro-addition.
The reactions of pentacoordinated silicon dihydrides with alcohols, carboxylic acids and carbonyl compounds have been studied.The dihydrides are markedly more reactive than the corresponding tetracoordinated species.
Reaction of fluorenone with 2 equiv. of Na in THF at room temp, gave the polymeric Na-fluorenone dianion complex 1 in 79% isolated yield, which upon reaction with 0.5 equiv. of [(C5Me5)IrCl(μ-Cl)]2 afforded the decarbonylation product 2; both 1 and 2 have been structurally characterized.
The catalytic efficiency of a Keplerate {Mo72V30} nanocapsule in the aerobic benzylic C-H oxidation of alcohols and hydrocarbons was exploited. The aerobic reactions were conducted using catalytic amounts of {Mo72V30} and under the co-catalytic effects of NHPI (N-hydroxyphthalimide) to furnish desired aldehydes and ketones in high yields and excellent selectivity. Different benzylic alcohols and hydrocarbons were shown to be amenable to oxidation under standard conditions presented here. Spectral data and leaching experiments revealed that the cluster had a long-term stability and could be used repeatedly in consecutive runs.
-
The decomposition of 9-diazofluorene (FlN2) brought about at a platinum anode or by chemical oxidants, Cu(BF4)2 or (p-BrC6H4)3N(+.)SbCl6(1-), in CH3CN solution leads to formation of mixtures of bifluorenylidene and flourenone azine in high yield.The reactions have been studied kinetically by conventional means and using the transient electrochemical techniques derivative linear sweep voltammetry (l.s.v.) and derivative cyclic voltammetry (d.c.v.).Constant-current electrolysis of FlN2 shows the characteristics of a chain process.The chemical oxidants give rise to a rate law -d/dt = k above a critical oxidant level, and the value of k is the same for both oxidants, even though their oxidation potentials are substantially different.It is inferred that the process studied is the chain propagation step in which the chain carrier reacts with a molecule of FlN2.Under the conditions of l.s.v. and d.c.v. experiments, it is shown that the instantaneously formed FlN2(+.) is consumed in a rapid process which is second order with respect to the cation radical and has a rate constant of 1.9 x 1E8 l mol-1 s-1; it is suggested that this process is cation radical dimerisation.Since the product of this step rapidly yields bifluorenylidene, it is argued that the slower macroscopic chain reactions do not involve dimerisation, but rather that FlN2(+.) rapidly attacks a further FlN2 molecule to generate the chain carrier which must have a lower oxidation potential than FlN2(+.).Possible structures for the chain carrier are considered in the light of earlier e.s.r. evidence for a radical intermediate.
The fluorenyl cation is a textbook example for a 4πantiaromatic cation. However, contrasting results have been published on how the annelated benzene rings compensate the destabilizing effect of the 4π antiaromatic five-membered ring in its core. Whereas previous attempts to synthesize this cation in superacidic media resulted in undefined polymeric material only, we herein report that it can be generated and isolated in amorphous water ice at temperatures below 30 K by photolysis of diazofluorene. Under these conditions, the fluorenylidene is protonated by water to give the fluorenyl cation, which could be characterized spectroscopically. Its absorption in the visible-light range matches that previously obtained by ultrafast absorption spectroscopy, and furthermore, its IR spectrum could be recorded. The IR bands in amorphous ice very nicely match predictions from DFT and DFT/MM calculations, suggesting the absence of strong interactions between the cation and surrounding water molecules.
Sodium aminodiboranate (NaNH 2(BH 3) 2, NaADBH) is a new member of the old borane family, which exhibits superior performance in chemoselective reduction. Experimental results show that NaADBH can rapidly reduce aldehydes and ketones to the corresponding alcohols in high efficiency and selectivity under mild conditions. There are little steric and electronic effects on this reduction.
A general and practical method for decarboxylative hydroxylation of carboxylic acids was developed through visible light-induced photocatalysis using molecular oxygen as the green oxidant. The addition of NaBH4 to in situ reduce the unstable peroxyl radical intermediate much broadened the substrate scope. Different sp3 carbon-bearing carboxylic acids were successfully employed as substrates, including phenylacetic acid-type substrates, as well as aliphatic carboxylic acids. This transformation worked smoothly on primary, secondary, and tertiary carboxylic acids.
A method for esterification of one or more carboxylic acid groups in a compound containing one or more carboxylic acid groups wherein the esterification reagent is a diazo-compound of formula: wherein the R1 and R2 groups of the diazo compound are selected such that the corresponding organic compound of formula: exhibits a —C—H pKa value between 18 and 29 as measured in DMSO. Specific reagents and methods for esterification are provided. The esterification reagents provided exhibit high selectivity for esterification of carboxylic acid groups over reaction with amine, alcohol or thiol groups in the compound containing one or more carboxylic acid groups. The method can be used to selectively esterify carboxylic acid groups in peptides or proteins.
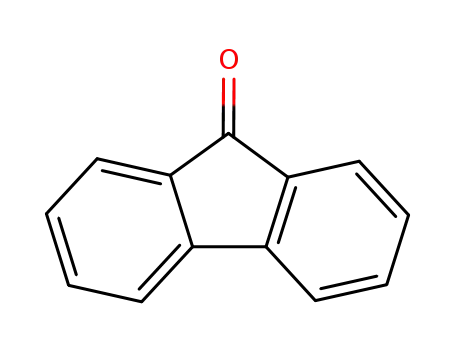
9-fluorenone

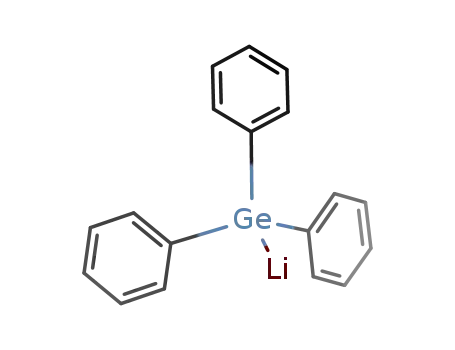
triphenyl germyllithium

9-Fluorenol

triphenylgermane

hexaphenyldigermane

chlorotriphenylgermane
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
tetrahydrofuran;
inert atmosphere; addn. of org. compd. soln. to soln. of Ge-compd. at 0°C, mixt. keeping at 0°C for 30 min, at room temp. for 2 h, warming (40°C, 1.5 h), soln. hydrolysis (HCl); soln. extn. (ether), drying (Na2SO4), soln. concn. (reduced pressure), redissoln. (THF); (1)H-NMR;
|
68% 33% <1 24% |

9-fluorenone


triphenyl germyllithium


9-Fluorenol


hexaphenyldigermane


chlorotriphenylgermane
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
diethyl ether;
inert atmosphere; addn. of Ge-compd. soln. to soln. of org. compd. at -10°C, keeping for 30 min, soln. allowing to stay at room temp. for 40 min, soln. hydrolysis (HCl); soln. extn. (ether), drying (Na2SO4), soln. concn. (reduced pressure); (1)H-NMR;
|
<1 60% 20% |
n-butyl magnesium bromide

9-fluorenone
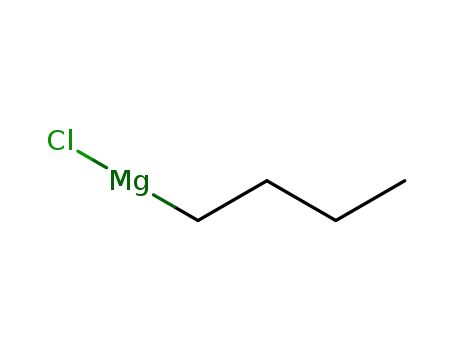
butyl magnesium bromide
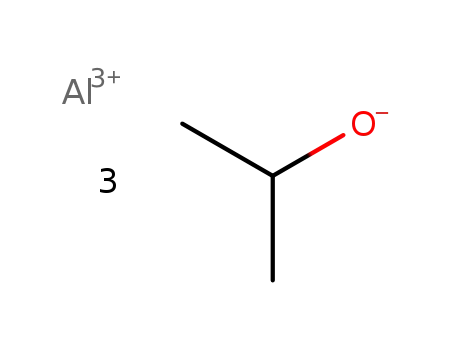
aluminum isopropoxide
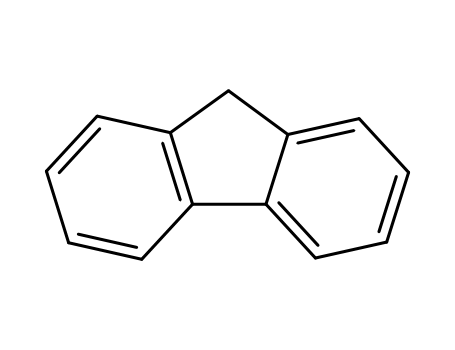
9H-fluorene
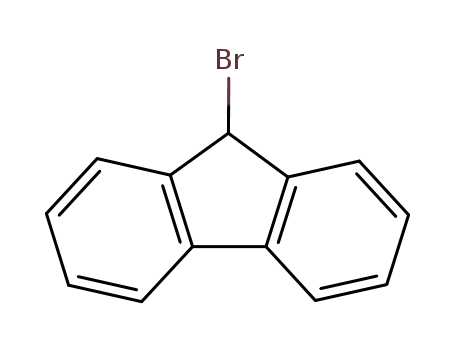
9H-fluoren-9-yl bromide

9-fluorenone
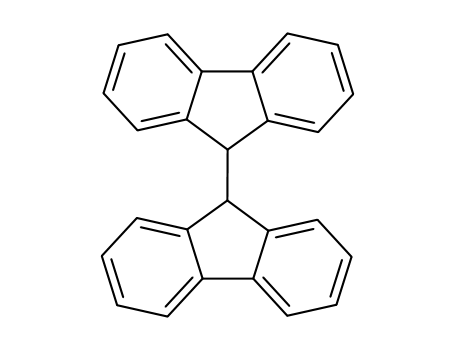
9,9'-bifluorenyl
CAS:14647-23-5
Molecular Formula:C26H24Cl2NiP2
Molecular Weight:528
CAS:2523-42-4
CAS:781-73-7