Your Location:Home >Products >OLED intermediates >Fluorenes >2523-42-4


Product Details
Chemical Properties
White powder
InChI:InChI=1/C13H9I/c14-11-5-6-13-10(8-11)7-9-3-1-2-4-12(9)13/h1-6,8H,7H2
A new strategy was proposed for the synthesis of polyphenol derivatives of fluorene-containing porphyrins to be used as the base for positive-tone photoresists for lithography with exposure at 13.5 nm wavelength, which allow fabrication of microchips with a size of down to 22 nm. Polyphenols based on fluorenecontaining porphyrins were synthesized for the first time. It was shown that these polyphenol derivatives can be used to obtain positive-tone photoresists with a resolution of 22 nm.
A small set of four new fluorenyl chromophores (5-5a-c) was accomplished by stepwise nucleophilic substitution, Friedel-Crafts acylation, Ullman coupling, aldol condensation and cyclization reactions. The fluorene moiety was substituted at 2,7,9 and 9′ positions with diverse groups. The synthesized derivatives were characterized by FTIR, 1H-NMR and 13C-NMR spectroscopic techniques. The optical properties were evaluated by by UV-VIS absorption and Fluorescence studies. HOMO and LUMO energy levels were evaluated by electrochemical studies and were found at ?5.37-5.83 eV and ? 2.47-2.94 eV respectively with band gap energy values 2.88 to 2.91 eV. The band gap energy values suggested that these synthesized molecules can be manipulated in the designing of blue and green OLEDS. [Figure not available: see fulltext.].
The requisition of small light emitting organic molecules is on the rise in the field of organic electronics. This study is aimed at synthesis of fluorene based chromophores which possess promising optoelectronic properties. The characterization of synthesized derivatives was carried out by spectroscopy (1H-NMR and 13C-NMR) and spectrometry (EI-MS). The optical properties evaluated by using UV-Vis spectroscopy, were from 394 to 420 nm, Fluorescence ranged 48 nm-113 nm, HOMO energy levels are ?6.75 eV to ?7.15 eV LUMO -4.08 to ?4.52eV with a band gap energy values 2.63–2.67 eV. The band gap energy values suggest that these synthesized molecules can be manipulated in the designing of blue and green OLEDS.
Inspired by the enol-degradation of luciferin, a new oxygen sensor with oppositely changed color and fluorescence has been designed. This new reaction-based dual mode sensor can not only be used as a highly selective instant "fluorescence on" oxygen probe, but also as a freshness indicator of food or food materials by using its property of time-adjustable color fading.
Caryl-F bond activation has become an important and quickly developing method for construction of carbon-based materials. We report that alumina-mediated C-F bond activation (AmCFA) enables construction of PAHs with zigzag periphery. This method includes
Diels–Alder cycloaddition of various dienophiles to the bay region of polycyclic aromatic hydrocarbons (PAHs) is a particularly effective and useful tool for the modification of the structure of PAHs and thereby their final properties. The Diels–Alder cycloaddition belongs to the single-step annulative π-extension (APEX) reactions and represents the maximum in synthetic efficiency for the constructions of π-extended PAHs including functionalised ones, nanographenes, and π-extended fused heteroarenes. Herein we report new applications of the APEX strategy for the synthesis of derivatives of 1,2-diarylbenzo[ghi]perylene, 1,2-diarylbenzo[ghi]perylenebisimide and 1,2-disubstituted-benzo[j]coronene. Namely, the so far unknown cycloaddition of 1,2-diarylacetylenes into the perylene and perylenebisimide bay regions was used. 1,2-Disubstituted-benzo[j]coronenes were obtained via cycloaddition of benzyne into 1,2-diarylbenzo[ghi]perylenes by using a new highly effective system for benzyne generation and/or high pressure conditions. Moreover, we report an unprecedented Diels–Alder cycloaddition–cycloaromatisation domino-type reaction between 1,4-(9,9-dialkylfluoren-3-yl)-1,3-butadiynes and perylene. The obtained diaryl-substituted core-extended PAHs were characterised by DFT calculation as well as electrochemical and spectroscopic measurements.
The invention discloses a preparation method of a photoelectric material intermediate, and is not reported in China at present. To the preparation method provided by the invention, the raw 2 material fluorene -9 is used as a series of operations such as single iodination and the C-N acenyman coupling, crude purification 9 and 9 - the like 4 -2 - 99.0%. The preparation method provided by the invention is simple to operate and suitable for industrial production. (by machine translation)
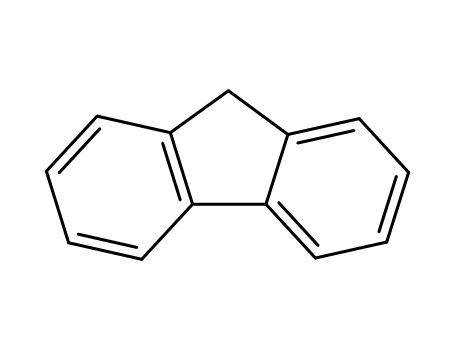
9H-fluorene


2-iodo-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
N,N,N-trimethylbenzenemethanaminium dichloroiodate; zinc(II) chloride;
In
acetic acid;
for 3h;
Ambient temperature;
|
94% |
|
With
iodine; acetic acid;
In
water;
at 80 ℃;
for 4h;
|
90% |
|
With
sodium chlorate; sulfuric acid; iodine; sodium thiosulfate;
In
methanol; water; acetic acid;
|
84.8% |
|
With
iodine; acetic acid; periodic acid;
In
water;
at 80 ℃;
for 4h;
Schlenk technique;
Inert atmosphere;
|
80% |
|
With
sulfuric acid; iodine; iodic acid;
In
water; acetic acid;
at 70 ℃;
for 1h;
|
78% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
water;
at 110 ℃;
for 4h;
|
78% |
|
With
iodine; periodic acid;
In
sulfuric acid; water; acetic acid;
at 60 - 65 ℃;
for 4h;
|
77% |
|
With
iodine; acetic acid; periodic acid;
In
water;
for 4h;
|
76% |
|
With
iodine; potassium carbonate; periodic acid;
In
sulfuric acid; water; acetic acid;
at 65 ℃;
for 4h;
|
72% |
|
9H-fluorene;
With
sulfuric acid; acetic acid;
In
water;
at 98 ℃;
With
iodine; periodic acid;
In
water;
at 65 ℃;
for 4h;
|
72% |
|
With
[bis(acetoxy)iodo]benzene; iodine;
at 20 ℃;
for 0.75h;
|
70% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
water;
for 4h;
Reflux;
|
70% |
|
With
iodic acid;
In
water;
|
65% |
|
With
iodine; acetic acid; periodic acid;
In
water;
at 80 ℃;
for 4h;
Inert atmosphere;
|
60% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
at 75 ℃;
for 2h;
|
58% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
at 65 ℃;
|
56% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
water;
at 70 ℃;
for 6h;
Inert atmosphere;
|
54% |
|
With
periodic acid dihydrate; sulfuric acid; iodine; acetic acid;
In
water;
at 70 ℃;
for 6h;
|
54% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
water;
at 70 - 100 ℃;
for 4h;
|
48% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
water;
at 70 ℃;
for 4h;
Heating;
|
48% |
|
With
sulfuric acid; iodine; nitric acid;
In
acetic acid;
at 35 ℃;
for 2h;
|
45% |
|
With
sulfuric acid; iodine; acetic acid; periodic acid;
In
water;
at 65 ℃;
|
30.2% |
|
With
iodine; acetic acid; periodic acid;
at 80 ℃;
for 4h;
|
|
|
With
sulfuric acid; iodine; iodic acid;
In
methanol; water;
at 60 ℃;
for 5h;
|
|
|
With
iodine; acetic acid;
|
|
|
With
sulfuric acid; iodine; periodic acid;
In
water; acetic acid;
|
|
|
With
iodine; periodic acid;
In
sulfuric acid; water; acetic acid;
at 70 ℃;
for 6h;
|
|
|
With
sulfuric acid; iodine; acetic acid;
In
water;
at 65 - 98 ℃;
for 4h;
|
24.6 g |
|
With
sulfuric acid; iodine; iodic acid; acetic acid;
In
water;
at 40 - 70 ℃;
for 1h;
|

2-aminofluorene


2-iodo-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; potassium iodide; sodium nitrite;
In
water;
at 5 ℃;
|
74% |
|
2-aminofluorene;
With
hydrogenchloride; sodium nitrite;
In
water;
at 0 ℃;
for 1h;
With
sodium iodide;
In
water;
at 20 ℃;
for 4h;
|
43% |

9H-fluorene
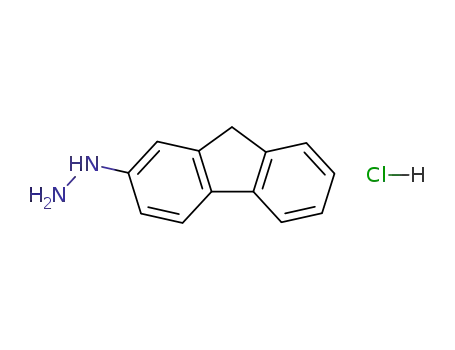
(9H-fluoren-2-yl)hydrazine hydrochloride

2-aminofluorene
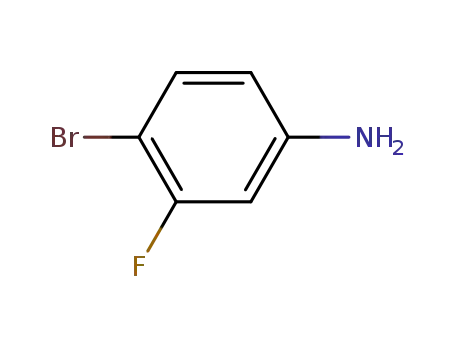
3-fluoro-4-bromophenylamine
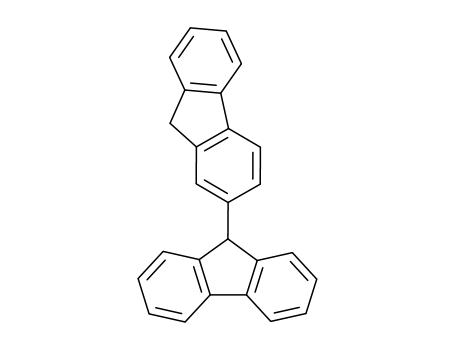
2-(9-Fluorenyl)-fluoren
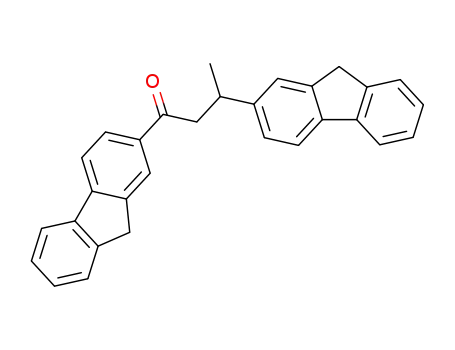
1,3-Di-(2-fluorenyl)-1-butanon

2-cyanofluorene
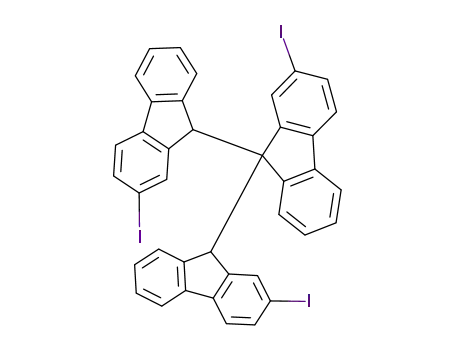
2,2',2''-Triiodo-9H,9''H-[9,9';9',9'']terfluorene
CAS:736928-22-6
CAS:1133-80-8
CAS:1689-64-1