Your Location:Home >Products >OLED intermediates >Fluorenes >1133-80-8

Product Details
Outline
2-bromofluorene is often used as synthetic intermediate of the OLED material in industry, by the reaction of barbier-type ultrasonic radiation, 2-bromofluorene is also used to study the conversion of aryl bromides to aryl triethoxy silane. Lab Preparation Methods: propylene carbonate is used as solvent, N-bromosuccinimide (NBS) is used as brominated reagent to carry bromination reaction of fluorine, and this reaction can synthesize 2-bromofluorene and 2,7-dibromofluorene. By the investigation of reaction temperature, NBS amount, the adding methods, the optimal reaction conditions for the synthesis of 2-bromofluorene is confirmed. The optimal reaction conditions are: reaction temperature is 23℃, the molar ratio of NBS with fluorine is 1.1:1, NBS is added by stage addition. First fluorene and propylene carbonate is preheated to 60℃, so fluorine is all dissolved, and then it drops to the reaction temperature, NBS is added by stage addition. 2-bromofluorene and 2,7-dibromofluorene recrystallizes with ethanol and acetic acid respectively, the purity of target product of liquid chromatography can reach more than 99%.
Uses
2-Bromofluorene is a nonintrusive end-capping reagent to control molecular weights and to generate well-defined oligomers. 2-Bromofluorene is used as a reagent for the preparation of dibromofluorene-labelled polystyrene by nitroxide-mediated polymerization. It is also being used in the agricultural sector as a fungicide for tomato disease control. Its carcinogenic effects on humans are currently being investigated.
Uses
(1) It can be used as pharmaceutical intermediate, it is also important intermediate in the synthesis of optoelectronic material. (2) By ultrasonic radiation of Barbier-type reaction, 2-bromofluorene is used for the study of aryl bromide (aryl bromides) converts to aryl triethoxysilane (aryltriethoxysilanes). It is also for the preparation of N-carbazole fluorene oligomers end-capped. It can be used as intermediates of OLED materials in industry.
Chemical Properties
white to slightly yellow crystalline powder
Uses
2-Bromofluorene was used in the synthesis of end-capping reagent 2-bromo-9,9-di-nhexylfluorene. It has been used in the preparation of poly(di-n-hexylfluorene)s end capped with 2-bromofluorene, 2-bromo-9,9-di-n-hexylfluorene and 9-bromoanthracene through Ni(0) mediated polymerization. It was also used in the synthesis of 2-bromo-9,9-dihexylfluorene.
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 4327, 1958 DOI: 10.1021/ja01549a053
General Description
2-Bromofluorene is a nonintrusive end-capping reagent to control molecular weights and to generate well-defined oligomers.
InChI:InChI=1/C13H9Br/c14-11-6-5-10-7-9-3-1-2-4-12(9)13(10)8-11/h1-6,8H,7H2
Organic tribophosphorescence materials are rarely reported and the introduction of Br atoms may be a practical way to design such materials. Here four bromine-substituted fluorene-based derivatives are presented and BrFlu?CBr, having fluorescence-phosphorescence dual-emission induced not only by UV light but also by mechanical stimulus, manifests the highest phosphorescence efficiency of 4.56 % upon photoirradiation. During the grinding process, three different triboluminescent spectra were identified. Upon introduction of a mechanical stimulus, the triboluminescence emission is cyan, whereas after an extended period it changed to blue. After removing the mechanical stimulus, green-white phosphorescent emission was observed. Careful research on single-crystal structures and theoretical calculations demonstrate that strong Br???Br interactions are vital to facilitate spin-orbit coupling and promote intersystem crossing, thus generating the unique properties.
Four new cross-conjugated small molecules based on a central benzo[1,2-d:4,5-d′]bisoxazole moiety possessing semi-independently tunable HOMO and LUMO levels were synthesized and the properties of these materials were evaluated experimentally and theoretically. The molecules were thermally stable with 5% weight loss occurring well above 350 °C. The cruciforms all exhibited blue emission in solution ranging from 433-450 nm. Host-guest OLEDs fabricated from various concentrations of these materials using the small molecule host 4,4′-bis(9-carbazolyl)-biphenyl (CBP) exhibited deep blue-emission with Commission Internationale de L'Eclairage (CIE) coordinates of (0.15 ≤ x ≤ 0.17, 0.05 ≤ y ≤ 0.11), and maximum luminance efficiencies as high as ~2 cd A-1. These results demonstrate the potential of benzobisoxazole cruciforms as emitters for developing high-performance deep blue OLEDs.
Highly luminescent network films on flat indium tin oxide (ITO) substrates are prepared by electropolymerization using an electroactive and fluorescent compound as precursor; the LEDs prepared using these films as a light emitting layer achieve the maximum luminance and external quantum efficiency of 4224 cd/m2 and 0.72%, respectively, which demonstrates that electrochemical synthesis can be a new route to construct the highly luminescent films. ; The Royal Society of Chemistry 2006.
Yamamoto or Suzuki-Miyaura coupling polymerizations of 2,3-diiodo-N- cyclohexylmaleimide with fluorene derivatives (2,7-dibromo-9,9′- dihexylfluorene and 9,9′-dihexylfluorene-2,7-diboronic acid) were carried out. The number-average molecular weights (Mn) of the resulting copolymers were 2600-3500 by gel permeation chromatography analysis. The fluorescence emission of the alternating copolymer showed the emission maxima at 551 nm in THF. On the other hand, the random copolymers showed the bimodal emission peaks at 418-420 and 555-557 nm region, respectively. The fluorescence peaks of the random copolymers on the long wavelength region (555-557 nm) were attributed to the conjugated neighboring N-cyclohexylmaleimide-9,9′- dihexylfluorene units in the polymer main chain. Furthermore, the copolymers exhibited the fluorescence solvatochromism by the difference of the polarity of solvents. The alternating and random copolymers showed the different fluorescence solvatochromism, and the emission colors are distinguishable by the naked eye, respectively. Copyright
-
A carbazole functionalized electro-active AIE-activity molecule, TPE-DFCz, was designed, synthesized, and well characterized. The clear difference in oxidation potentials between tetraphenylethylene (TPE) unit and carbazole groups was found which guaranteed that polymerization occurred only at the peripheral carbazole groups and the TPE unit remained unchanged. Its luminescent network film was prepared conveniently by electrochemical polymerization (EP). The cross-linked film exhibited green emission with high quantum efficiency of 63%, relatively smooth surface, and good thermal stability. The effect of different scan cycles on the optical property was also investigated. The electroluminescent device using the optimized polymer film as active layer showed a maximum luminance of 3200 cd?m?2 and a maximum luminance efficiency of 1.16 cd?A?1 with very low roll-off of the efficiency. The AIE-active EP films afford more opportunities to develop polymer films with high quantum efficiency via a simple, effective method and promote the potential applications in display devices.
A mild, simple, and efficient synthetic procedure for the preparation of 2-monobromo-, 2,7-dibromo-, and 2,2′,7,7′-tetrabromo-substituted spirobifluorene derivatives and their key intermediates, 2-monobromo- and 2,7-dibromo-substituted fluorene compounds, has been developed. The oxidative bromination of fluorene and spirobifluorene was achieved using NaBr/H2O2 as the bromine source. High conversion of the starting materials was achieved together with good selectivities under optimized reaction conditions. Copyright Taylor & Francis Group, LLC.
A new type of oligo(phenylenevinylene) dimer, 2,5,2,5- tetra(9,9-dihexylfluorenyl)biphenyl (TFB), with a biphenyl linkage center and four fluorene end groups, has been synthesized by the Wittig reaction. The full characterization of its structure and optical properties, as well as the performance of its electroluminescent devices are presented. TFB shows strong blue fluorescence both in solution and as a solid film. High-quality films of TFB for light-emitting devices (LEDs) can be fabricated both by vacuum evaporation and the spin-coating technique, which is very special and interesting. Single-layer and multi-layer light-emitting devices using TFB as the active layer all show efficient blue emission.
Two fluorene-based boronic acids, 9,9-dimethyl-9H-fluoren-2-yl-2-boronic acid (1) and 9,9-dimethyl-9H-fluoren-2,7-diyl-2,7-diboronic acid (2), were synthesized and their sensing abilities for detection of D-monosaccharides were investigated by fluorescence at physiological pH. It was found that both boronic acids 1 and 2 have high selectivity and sensitivity for D-fructose with stability constant of 47.2 and 412.9, respectively. The sensor 2 showed a linear response toward D-fructose in the concentration range from 5 × 10-5 to 10-1 mol L-1 with the detection limit of 2 × 10-5 mol L-1.
-
A series of alternating copolymers (PT-BDFQx, PC-BDFQx and PBDT-BDFQx) have been synthesized bearing novel planar bis(9,9-di(2-ethylhexyl)-9H-fluoren-2-yl)quinoxaline (BDFQx) as acceptor unit, using benzo[1,2-b:4,5-b′]dithiophene (BDT), thiophene (T) and carbazole (C) as donor units via Stille or Suzuki coupling reactions. XRD characterization indicated that the presence of planar BDFQx unit (monomer 8) is favorable for the promotion of crystallization in the solid state and GPC results illustrated that the import of multiple chains in planar BDFQx unit raises the polymers molecular weight. Electrochemical measurement results suggested that three copolymers possess deep HOMO energy level of -5.50-5.77 eV. The polymer solar cell with structure of ITO/PEDOT:PSS (30 nm)/polymer: PCBM (60 nm)/Bphen (10 nm)/Ag (100 nm) exhibited the highest Voc of 0.80 V with PBDT-BDFQx as p-type polymer, while the best power conversion efficiency (PCE) of 0.9% was obtained using a blend of PBDT-BDFQx and PCBM(1:4) as active layer.
Over the past 20 years, ruthenium(II)-based dyes have played a pivotal role in turning dye-sensitized solar cells (DSCs) into a mature technology for the third generation of photovoltaics. However, the classic I3-/I- redox couple limits the performance and application of this technique. Simply replacing the iodine-based redox couple by new types like cobalt(3+/2+) complexes was not successful because of the poor compatibility between the ruthenium(II) sensitizer and the cobalt redox species. To address this problem and achieve higher power conversion efficiencies (PCEs), we introduce here six new cyclometalated ruthenium(II)-based dyes developed through ligand engineering. We tested DSCs employing these ruthenium(II) complexes and achieved PCEs of up to 9.4% using cobalt(3+/2+)-based electrolytes, which is the record efficiency to date featuring a ruthenium-based dye. In view of the complicated liquid DSC system, the disagreement found between different characterizations enlightens us about the importance of the sensitizer loading on TiO2, which is a subtle but equally important factor in the electronic properties of the sensitizers.
The invention relates to a conjugated diene monomer 9-9 - dialkyl fluorene as a structural unit and a synthesis method thereof, and belongs to the technical field of organic synthesis. 9, 9 - Dialkyl fluorene is the structural unit of the conjugated diene monomer. Herein R is a linear or branched alkyl group having a different number (6 - 30) of carbon atoms, n Shows functional groups of each intermediate, and a functional ethylenic monomer with Suzuki and 9 dialkyl fluorene as a core with different conjugation lengths can be obtained by repeating the bromination and acetylation reduction of the fluorene or 9 - fluorene oligomer. The problem of flexible expansion of chemical reaction of organic small molecules of fluorene units can be solved.
The invention discloses a fluorene derivative with AIE characteristic. The structural formula of the derivative is shown in the specification. In the formula, R =- CHO, -COOH, -Br, -I, -NO2, -NH2, -OCH3, -CH3, -CN, -NHCOCH3, -CF3 or -CCl3. The fluorene derivative can be used as a fluorescent probe for identifying small biological molecules, has efficient specific identification on tryptophan (the detection limit is 1.15 [mu]M), and can also be used for detecting small molecular substances such as explosives, anions, metal cations (including rare earth metals) and the like.
Caryl-F bond activation has become an important and quickly developing method for construction of carbon-based materials. We report that alumina-mediated C-F bond activation (AmCFA) enables construction of PAHs with zigzag periphery. This method includes
The invention discloses a bipolar small molecular luminescent material capable of being processed by adopting an environment-friendly solvent and taking a naphtho-indenofluorene unit as a core as well as a production method and application of the bipolar small molecular luminescent material. The production method comprises the following steps: by taking the naphtho-indenofluorene unit containing a polar substituent group as the core; carrying out a Suzuki coupling reaction, and connecting an electron donor unit and an electron withdrawing unit onto two sides of the naphtho-indenofluorene unit in sequence, so as to obtain the bipolar small molecular luminescent material taking the naphtho-indenofluorene unit as the core. The bipolar small molecular luminescent material taking the naphtho-indenofluorene unit as the core, disclosed by the invention, has good solubility, film forming property and thin film form stability in the environment-friendly solvent; a luminescent layer produced by the luminescent material can avoid a mixing phenomenon with a hole/electron transmission layer interface; the prepared luminescent layer does not need to be subjected to annealing treatment when being used for preparing a luminescent device, so that a preparation process is simple.
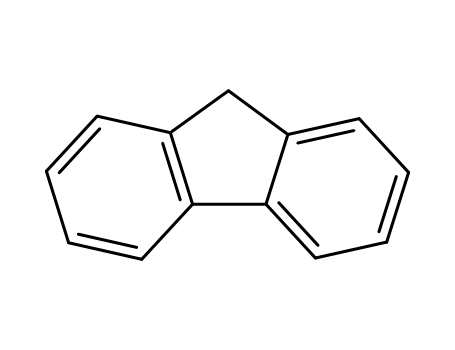
9H-fluorene


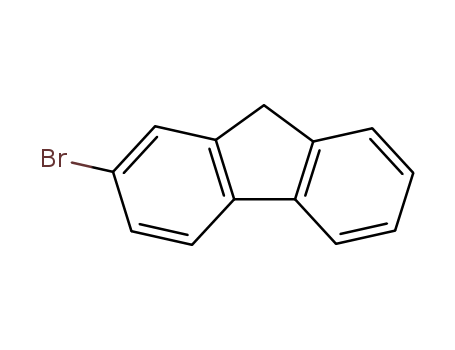
2-bromo-9H-fluorene

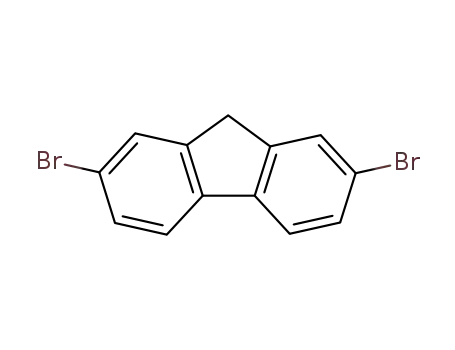
2,7-dibromo-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; dihydrogen peroxide; sodium bromide;
In
water; 1,2-dichloro-ethane;
at 20 ℃;
for 10h;
|
33% |
|
bei der Bromierung;
|

9H-fluorene


2-bromo-9H-fluorene
| Conditions | Yield |
|---|---|
|
With
mono(N,N,N-trimethylbenzenaminium) tribromide; zinc(II) chloride;
In
acetic acid;
for 0.5h;
Ambient temperature;
|
99% |
|
With
N-Bromosuccinimide;
In
1,2-propylene cyclic carbonate;
at 60 ℃;
for 1h;
|
95% |
|
With
iron(III) chloride; bromine;
In
chloroform;
at 0 ℃;
for 3h;
Darkness;
Inert atmosphere;
|
92% |
|
With
N-Bromosuccinimide;
In
various solvent(s);
at 20 ℃;
for 0.5h;
|
91% |
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
at 60 ℃;
|
91% |
|
9H-fluorene;
With
hydrogen bromide; dibromotriphenylphosphorane; dibenzoyl peroxide;
at 65 ℃;
for 1.5h;
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione;
at 25 ℃;
for 4h;
Reagent/catalyst;
Temperature;
|
91.35% |
|
With
sodium bromate; hydrogen bromide; trimethylbenzylammonium bromide;
In
dichloromethane; water;
at 20 - 30 ℃;
for 2h;
under 760.051 Torr;
Temperature;
Reagent/catalyst;
|
90% |
|
With
iron(III) chloride; N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
Darkness;
|
89.8% |
|
With
bromine;
In
dichloromethane;
|
87% |
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; sulfuric acid;
In
dichloromethane;
|
86% |
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
at 40 - 50 ℃;
for 10h;
|
85% |
|
With
N-Bromosuccinimide; trityl tetrafluoroborate;
In
dichloromethane; acetonitrile;
at 20 ℃;
chemoselective reaction;
Inert atmosphere;
|
85% |
|
With
bromine; iron;
In
chloroform;
at 5 - 20 ℃;
for 6h;
|
84% |
|
With
bromine; iron;
In
chloroform;
at 5 ℃;
for 16h;
|
84% |
|
With
bromine; iron;
In
chloroform;
at 5 - 20 ℃;
for 6h;
Cooling with ice;
|
84% |
|
With
bromine; iron;
In
chloroform;
at 5 ℃;
Cooling with ice;
|
84% |
|
With
bromine; iron;
In
chloroform;
at 5 ℃;
for 16h;
Cooling with ice;
|
84% |
|
With
N-Bromosuccinimide;
In
acetone;
for 3h;
Reflux;
|
83% |
|
With
iron(III) chloride; N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
for 24h;
Inert atmosphere;
Darkness;
|
80% |
|
With
N-Bromosuccinimide;
In
1,2-propylene cyclic carbonate;
at 60 ℃;
for 1h;
Inert atmosphere;
|
73% |
|
With
hydrogen bromide; dihydrogen peroxide;
In
dichloromethane; water;
at 10 - 20 ℃;
for 15h;
|
73% |
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
at 20 - 60 ℃;
Inert atmosphere;
|
71% |
|
With
N-Bromosuccinimide; thioacetamide;
In
acetonitrile;
at 20 ℃;
for 19h;
regioselective reaction;
|
71% |
|
With
N-Bromosuccinimide; acetic acid;
at 40 ℃;
for 5h;
Temperature;
|
71% |
|
With
1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; trifluorormethanesulfonic acid;
In
dichloromethane;
at 30 ℃;
for 1h;
|
62% |
|
With
N-Bromosuccinimide; iron(III) chloride;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
56% |
|
With
bromine;
|
32% |
|
bei der Bromierung;
|
|
|
With
bromine;
|
|
|
With
1,2-propylene cyclic carbonate; bromine;
|
|
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
|
|
|
With
chloroform; bromine;
|
|
|
With
chloroform; bromine;
|
|
|
With
bromine;
at 113 - 115 ℃;
|
|
|
With
N-Bromosuccinimide; boron trifluoride diethyl etherate; benzene;
|
|
|
With
bromine;
|
|
|
With
bromine;
In
1,4-dioxane;
|
|
|
With
N-Bromosuccinimide; iron(III) chloride;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
|
|
With
sodium hydrogen sulphite; sulfuric acid; bromine;
In
water; toluene;
|
|
|
With
N-Bromosuccinimide;
In
1,2-propylene cyclic carbonate;
at 60 ℃;
for 1.5h;
|
|
|
With
N-Bromosuccinimide;
In
1,2-propylene cyclic carbonate;
at 60 ℃;
|
|
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
at 60 ℃;
Inert atmosphere;
|
|
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
|
|
|
With
1,2-propylene cyclic carbonate; N-Bromosuccinimide;
at 60 ℃;
|
|
|
With
N-Bromosuccinimide;
at 20 ℃;
|
|
|
With
iron(III) chloride; N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
|
|
With
N-Bromosuccinimide;
In
acetone;
at 60 ℃;
for 8h;
|
|
|
With
N-Bromosuccinimide; dibenzoyl peroxide;
In
1-methyl-pyrrolidin-2-one;
at 60 ℃;
for 7h;
Temperature;
|
|
|
With
1-methyl-pyrrolidin-2-one; N-Bromosuccinimide; dibenzoyl peroxide;
at 60 ℃;
for 7h;
Temperature;
|
|
|
With
N-Bromosuccinimide;
In
acetone;
|
|
|
With
iron(III) chloride; bromine;
In
chloroform;
at 0 ℃;
for 3h;
|
1,4-dioxane

9H-fluorene
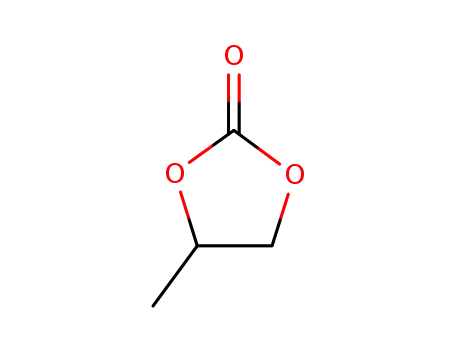
1,2-propylene cyclic carbonate
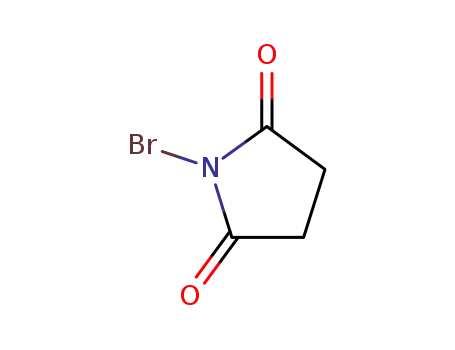
N-Bromosuccinimide

9H-fluorene
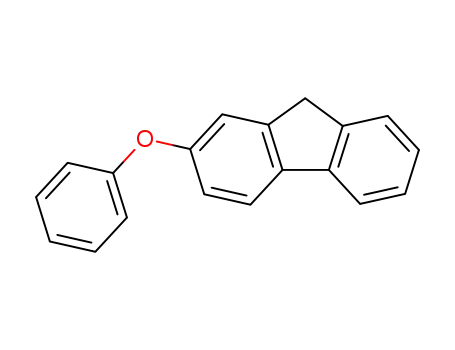
fluoren-2-yl-phenyl ether
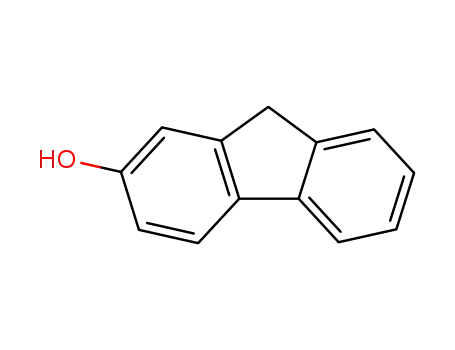
2-hydroxyfluorene

2-aminofluorene
CAS:3460-18-2
CAS:131409-18-2
CAS:2523-42-4