Your Location:Home >Products >OLED intermediates >Fluorenes >201138-91-2

Product Details
Cobalt corroles with different acid/base pendants, LBr-Co, LCOOH-Co, LPO(OH)2-Co, and LCH2PO(OH)2-Co (L = 5,15-bis-(pentafluorophenyl)-10-(4-dibenzofuran)corrole), were synthesized and examined as catalysts for oxygen and hydrogen evolution from neutral aqueous solutions. Co corroles with phosphonic acid pendants showed improved activities in both processes, highlighting the importance of the secondary coordination sphere in catalyst design.
A novel catalyst-free approach to benzoannulated oxygen-containing heterocycles from fluorinated oligophenylenes is reported. Unlike existing methods, the presented reaction does not require an oxygen-containing precursor and relies on an external oxygen source, potassium tert-butoxide, which serves as an O2? synthon. The radical nature of the reaction facilitates nucleophilic substitution even in the presence of strong electron-donating groups and enables de-tert-butylation required for the complete annulation. Also demonstrated is the applicability of the method to introduce five-, six-, and seven-membered rings containing oxygen, whereas multiple annulations also open up a short synthetic path to ladder-type O-heteroacenes and oligodibenzofurans.
The homogeneous, gas-phase oxidative thermal degradation of 2-bromophenol was studied in a 1 cm i.d., fused silica flow reactor at a concentration of 88 ppm, reaction time of 2.0 s, over a temperature range from 300 to 1000 °C. Observed products in order of yield were dibenzo-p-dioxin (DD) > 4,6-dibromodibenzofuran (4,6-DBDF) > 4-monobromodibenzofuran (4-MCDF), dibenzofuran (DF), 1-monobromodibenzo-p-dioxin (1-MBDD), naphthalene, bromonaphthalene, 2,4-dibromophenol, 2,6-dibromophenol, phenol, bromobenzene, and benzene. This result is in contrast to the oxidation of 2-chlorophenol, where the major product is 4,6-dichlorodibenzofuran (4,6-DCDF). 4,6-DBDF was observed in high yields in contrast to our previous results for the pyrolysis of 2-bromophenol, where 4,6-DBDF was not detected. The increase in 4,6-DBDF yields is attributed to hydroxyl radical being the major chain carrier under oxidative conditions, which favors hydrogen-abstraction reactions that lead to formation of 4,6-DBDF. However, DD is still the highest yield product under oxidative conditions because of the relative ease of displacement of Br? in the ring-closure reaction.
The syntheses of six new host systems are reported whose semirigid, saddle-shaped structures are based on incorporation of three to four dibenzofuran units into a macroring (4-9). The two clefts in 4 have long axes mutually perpendicular to one another, e
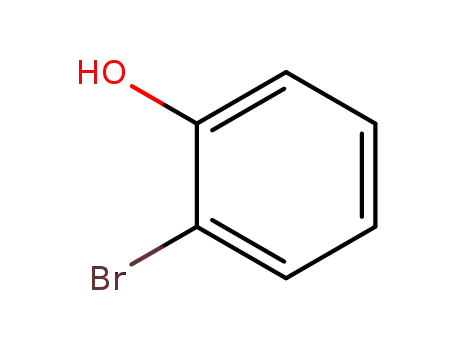
2-hydroxybromobenzene

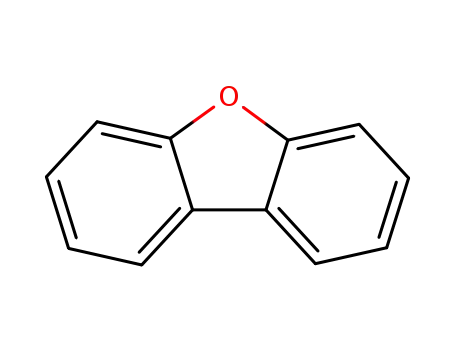
dibenzofuran

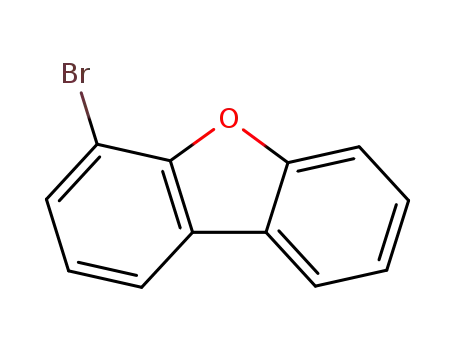
4-bromodibenzofuran

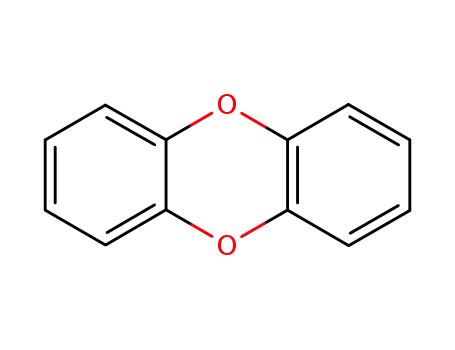
dioxin

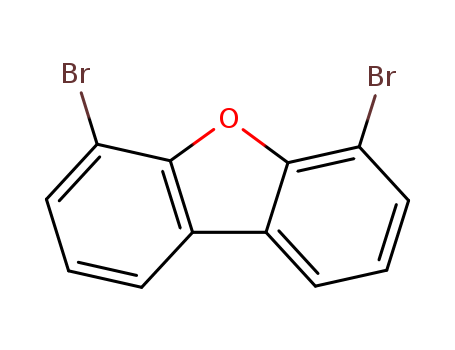
![4,6-dibromodibenzo-[b,d]furan](/upload/2023/2/93ab97a9-f175-445d-a7b0-dca8ba264ccf.png)
4,6-dibromodibenzo-[b,d]furan
| Conditions | Yield |
|---|---|
|
With
oxygen;
at 650 ℃;
for 0.000555556h;
under 760 Torr;
Further Variations:;
Temperatures;
Product distribution;
|
16.6% 2.4% 0.9% 0.82% |
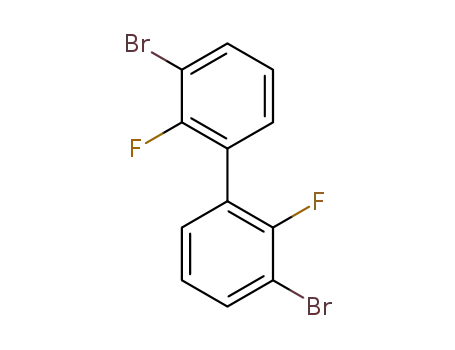
3,3’-dibromo-2,2’-difluoro-1,1’-biphenyl

![4,6-dibromodibenzo-[b,d]furan](/upload/2023/2/93ab97a9-f175-445d-a7b0-dca8ba264ccf.png)
4,6-dibromodibenzo-[b,d]furan
| Conditions | Yield |
|---|---|
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide; potassium tert-butylate;
at 120 ℃;
for 3h;
Microwave irradiation;
|
92% |

dibenzofuran

2-hydroxybromobenzene
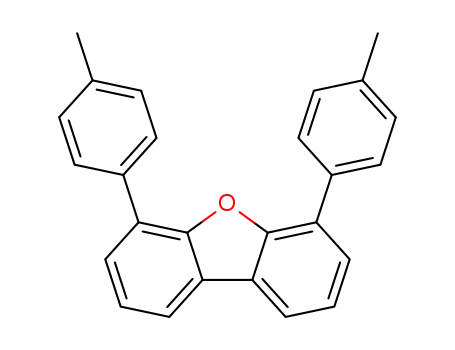
4,6-bis(p-tolyl)dibenzo[b,d]furan
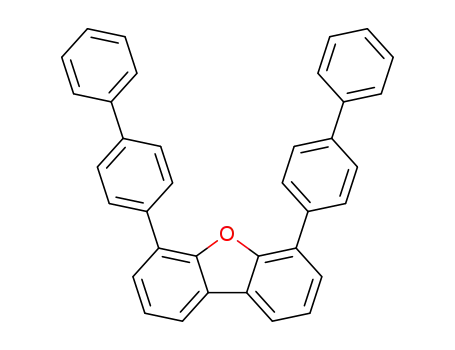
4,6-bis(biphenyl-4-yl)dibenzo[b,d]furan
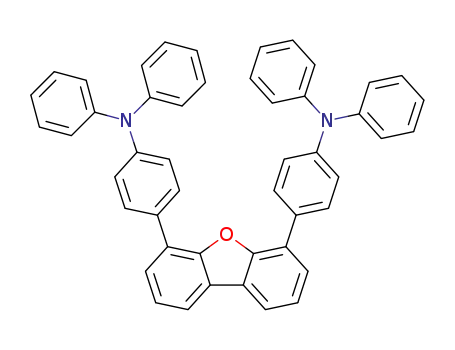
4,6-bis[4-(diphenylamino)phenyl]dibenzo[b,d]furan
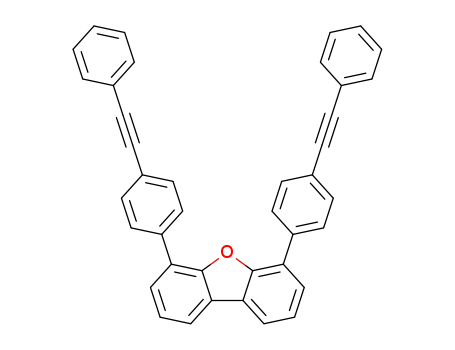
4,6-bis[4-(phenylethynyl)phenyl]dibenzo[b,d]furan
CAS:605644-46-0
CAS:34172-50-4
CAS:19261-06-4