Your Location:Home >Products >Organic phosphines >Other organic phosphines >554-70-1
Product Details
Chemical Properties
Colorless Transparent Liquid
Uses
In organic syntheses.
Uses
suzuki reaction
Uses
Triethylphosphine is used as a ligand for arylation. It is used in organic synthesis.
Safety Profile
Highly flammable liquid. Reactswith oxygen at low temperatures to form an explosiveproduct. When heated to decomposition it emits toxicfumes of POx.
Purification Methods
All operations should be carried out in an efficient fume cupboard because it is flammable, toxic and has a foul odour. Purify the phosphine by fractional distillation at atmospheric pressure in a stream of dry N2, as it is oxidised by air to the oxide. In 300% excess of CS2 it forms Et3PCS2 (m 118-120o crystallising from MeOH) which decomposes in CCl4 to give Et3PS as a white solid m 94o when recrystallised from EtOH. [Sorettas & Isbell J Org Chem 27 273 1962, Henderson & Streuli J Am Chem Soc 82 5791 1960, pK: Henderson & Streuli J Am Chem Soc 82 5791 1960, see also trimethylphosphine.] Store it in sealed vials under N2. Alternatively, dissolve it in Et2O and shake it with a solution of AgI and KI to form the insoluble complex. Filter off the complex, dry it over P2O5 and the Et3P is regenerated by heating the complex in a tube attached to a vacuum system. [Hewitt & Holliday J Chem Soc 530 1953, Schettas & Isbell J Org Chem 27 2573 1962, Kosolapoff Organophosphorus Compounds, Wiley p 31 1950, Beilstein 4 IV 3431.]
InChI:InChI=1/C6H15P/c1-4-7(5-2)6-3/h4-6H2,1-3H3
The complexes (η5-C5H5)NiM3(μ-H)3(CO)(9-n)Ln (M = Ru, Os, n = 1.2: L = PPh3, PPh2H, PCy3, PEt3) and M3(CO)(12-n)Ln (M = Ru, n = 1-3: L = PPh3, PPh2H, PCy3, PEt3; M = Os, n = 1,2: L = PPh3) have been synthesized by new or known procedures.Reacti
Given its earth abundance, silicon is ideal for constructing Lewis acids of use in catalysis or materials science. Neutral silanes were limited to moderate Lewis acidity, until halogenated catecholato ligands provoked a significant boost. However, catalytic applications of bis(perhalocatecholato)silanes were suffering from very poor solubility and unknown deactivation pathways. In this work, the novel per(trifluoromethyl)catechol, H2catCF3, and adducts of its silicon complex Si(catCF3)2 (1) are described. According to the computed fluoride ion affinity, 1 ranks among the strongest neutral Lewis acids currently accessible in the condensed phase. The improved robustness and affinity of 1 enable deoxygenations of aldehydes, ketones, amides, or phosphine oxides, and a carbonyl-olefin metathesis. All those transformations have never been catalyzed by a neutral silane. Attempts to obtain donor-free 1 attest to the extreme Lewis acidity by stabilizing adducts with even the weakest donors, such as benzophenone or hexaethyl disiloxane.
Reduction of phosphine oxides into the corresponding phosphines using PhSiH3 as a reducing agent and Ph3C+[B(C6F5)4]? as an initiator is described. The process is highly efficient, reducing a broad range of secondary and tertiary alkyl and arylphosphines, bearing various functional groups in generally good yields. The reaction is believed to proceed through the generation of a silyl cation, which reaction with the phosphine oxide provides a phosphonium salt, further reduced by the silane to afford the desired phosphine along with siloxanes. (Figure presented.).
Investigations on the boundaries between the neutral and cationic models of (Mesityl)2EX (E = Sb, Bi and X = Cl?, OTf?) have facilitated reversing the Lewis acidity from bismuth to antimony. We use this concept to demonstrate a higher efficiency of (Mesityl)2SbOTf(Mesityl)2BiOTf in the catalytic reduction of phosphine oxides to phosphines. The experiments supported with computations described herein will find use in designing new Lewis acids relevant to catalysis.
The invention provides a method for producing alkyl phosphine, and specifically relates to a method for producing triethyl phosphine. The method comprises the following steps: mixing hydrogen phosphide with ethylene in a solvent, adding a photocatalyst, and carrying out a reaction for 1-12 h under illumination of 20-50 W to obtain triethyl phosphine, wherein the reaction temperature being 0-50 DEGC. According to the invention, under an illumination condition, the photocatalyst easily induces a radical reaction to enhance the reaction activity of hydrogen phosphide and olefin so as to substantially improve the reaction efficiency, the rapid reaction can be performed at the room temperature and the normal pressure, the reaction time is short, the pollution and the toxicity of the catalyst are low, the toxicity of the used solvent is low compared to the toxicity of toluene and the like commonly used in the prior art, the post-treatment is relatively simple, and the yield and the purity of the product are high.
triethylbenzyl phosphonium chloride

benzyl chloride

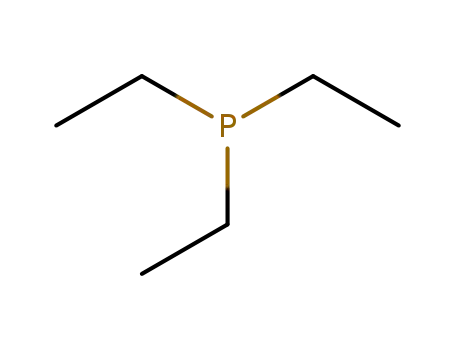
triethylphosphine
| Conditions | Yield |
|---|---|
|
at 340 ℃;
|
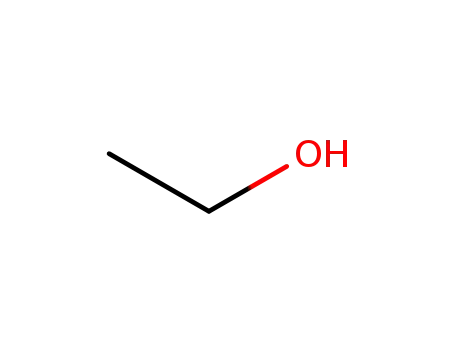
ethanol

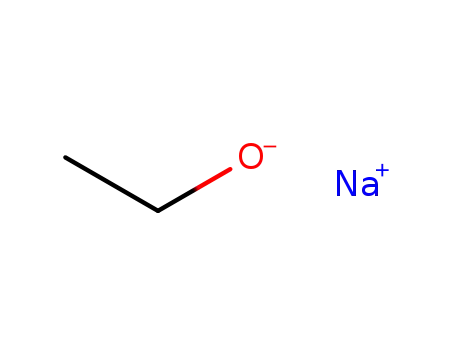
sodium ethanolate

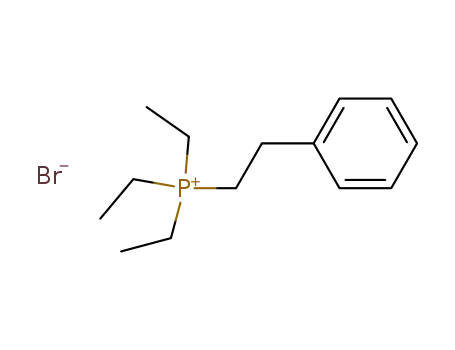
triethyl-phenethyl-phosphonium; bromide

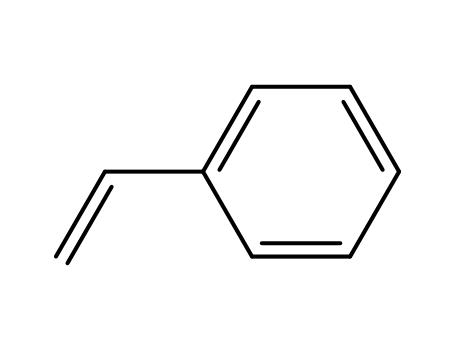
styrene

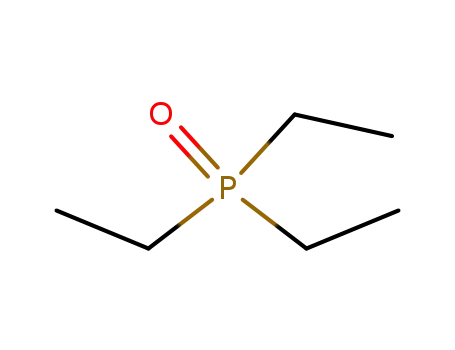
triethylphosphine oxide

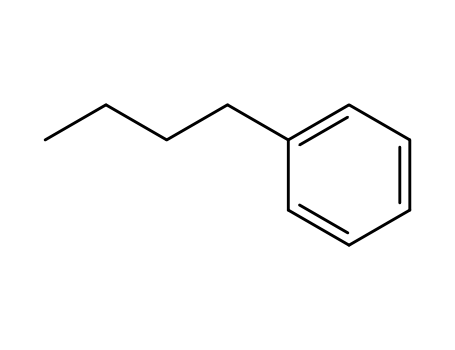
1-butylbenzene

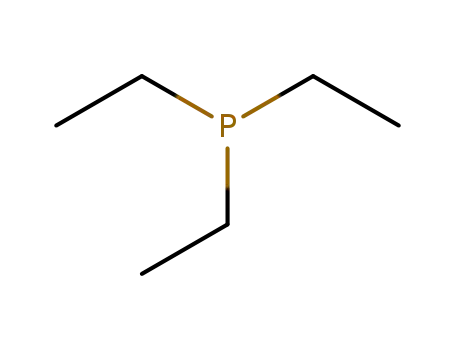
triethylphosphine
| Conditions | Yield |
|---|---|
|
beim anschliessenden Erhitzen unter Stickstoff;
|
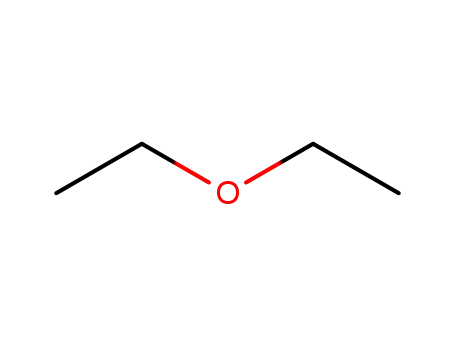
diethyl ether
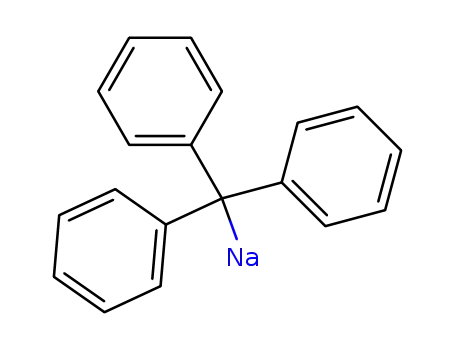
triphenylmethyl sodium
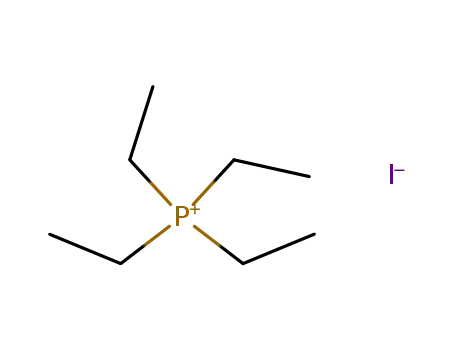
tetraethylphosphonium iodide

ethanol
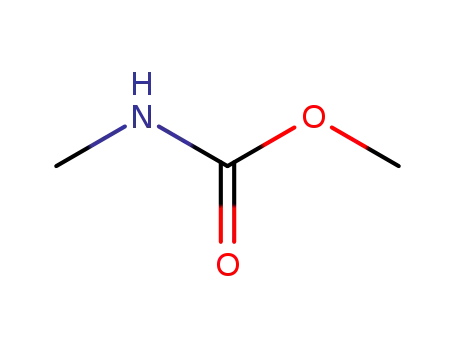
methyl N-methylcarbamate
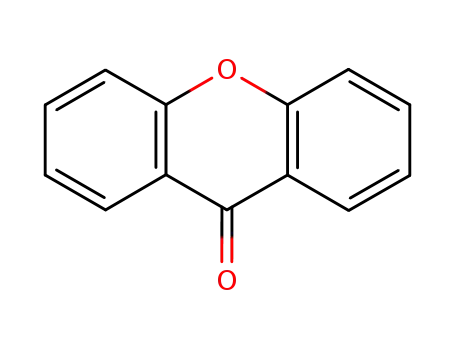
xanth-9-one
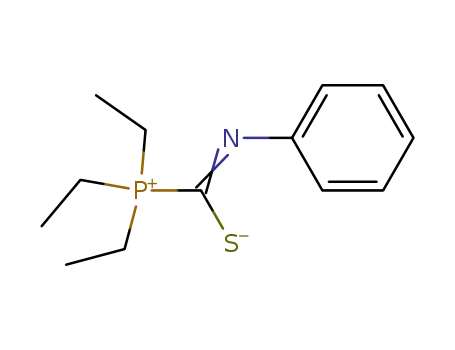
triethyl-phenylthiocarbamoyl-phosphonium betaine
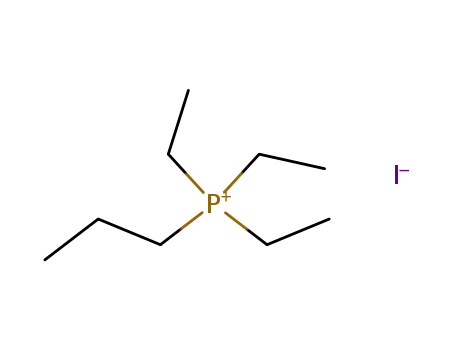
triethyl-propyl-phosphonium; iodide
CAS:76189-56-5
CAS:65344-26-5
CAS:128363-76-8
CAS:5518-52-5