Your Location:Home >Products >Organic phosphines >Other organic phosphines >128363-76-8
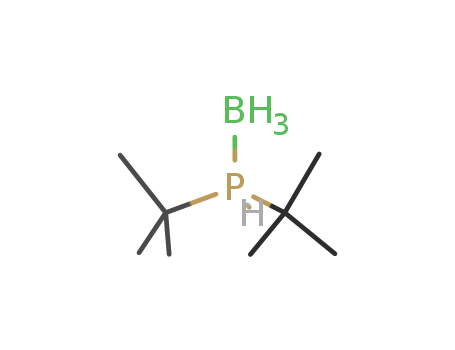
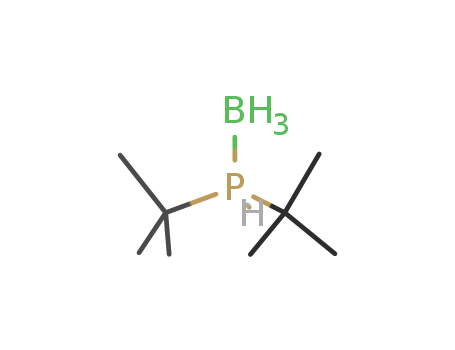
Product Details
Chemical Properties
white crystalline needles
Uses
Reactant for:Preparation of N-heterocyclic carbene borane complexes by Lewis base exchange reactionsDehydrocoupling reactionsTele-substitution reactions on fluorobenzenechromium complexesPalladium-catalyzed biaryl-coupling reactionsPalladium-catalyzed Suzuki coupling
The PNS pincer-type ligand 1 and the novel Ru(PNS) complexes 2-8 were synthesized and characterized. The (PNS)RuH(Cl)CO complex 2 was prepared by reaction of ligand 1 with RuH(Cl)CO(PPh3)3. 2 reacted with KHMDS (potassium bis(trimethylsilyl)amide) to form the symmetrical dimeric complex 4 via the intermediacy of the dearomatized complex (PNS*)Ru(H)CO 3, in which deprotonation of the benzylic-S arm took place. Reaction of 2 with excess NaH gave the dimeric 4, by a formal intermolecular attack of the benzylic arm on a second ruthenium center. Complex 4 underwent spontaneous transformation in solution to the dinuclear complex 5 via C-S bond cleavage, resulting in the loss of a S-bound tBu group. Treatment of 2 with KHMDS in the presence of PEt3 resulted in the trapping of intermediate 3 in the form of the dearomatized complex 8. Reaction of 2 with LiHBEt3 gave the trans-dihydride complex 6, which reacted with CO2 to give the formato complex 7, in which the formato ligand is located trans to the hydride. Complexes 2, 4, and 5 were also investigated as catalysts for the dehydrogenative coupling of alcohols with amines.
The dehydrocoupling of the sterically hindered phosphine-borane adduct tBu2PH·BH3 above 140 °C is catalyzed by the rhodium complexes [Rh(1,5-cod)2:][OTf] or Rh6(CO)16 to give the four-membered chain tBu2/s
The first example of highly enantioselective fluoroarylation of gem-difluoroalkenes with aryl halides is presented by using a new chiral sulfinamide phosphine (Sadphos) type ligand TY-Phos. N-Me-TY-Phos can be easily synthesized on a gram scale from readily available starting materials in three steps. Salient features of this work including readily available starting materials, good yields, high enantioselectivities as well as broad substrate scope make this approach very practical and attractive. Notably, the asymmetric synthesis of an analogue of a biologically active molecule is also reported.
The purpose of the present invention is to provide an N,N-bis(2-dialkylphosphinoethyl)amine-borane complex which is a ruthenium complex that exhibits excellent catalytic activity in a hydrogenation reaction, etc., and a production method therefor, and a method for efficiently producing a ruthenium complex containing N,N-bis(2-dialkylphosphinoethyl)amine as a ligand. The present invention is capable of efficiently producing an amine-borane complex (3) by reacting an oxazolidinone compound (1) with a dialkylphosphine-borane compound (2) in the presence of a base. The present invention is also capable of efficiently producing a ruthenium complex (5) by reacting the amine-borane complex (3) with a ruthenium compound (4) in the presence of an amine. (In the formula, a solid line, a dashed line, B, C, H, L1-L3, LG, n, N, O, P, Ru, X, and R1-R10 are as defined in the description.)
The invention discloses a cobalt complex, a preparation method thereof, and an application thereof in the selective catalysis of a transfer hydrogenation reaction of a cyano group. The structural formula of the cobalt complex is represented by formula I. The cobalt complex is prepared through a reaction of a cobalt salt and an NNP ligand or a PNP ligand under the protection of an inert atmosphere;and the chemical formula of the cobalt salt is CoX12, wherein X1 represents halogen, a sulfate radical, a perchlorate radical, a hexafluorophosphate radical, a hexafluoroantimonate radical, a tetrafluoroborate radical, a trifluoromethanesulfonate radical or a tetra(pentafluorophenyl)borate radical. The cobalt complex can be used in the selective catalysis of the transfer hydrogenation reaction ofthe cyano group to obtain a primary amine compound, a secondary amine compound and a tertiary amine compound, the primary amine compound, the secondary amine compound and the tertiary amine compoundare important intermediates in a series of subsequent functionalizing reactions, and the cobalt complex has a very high catalysis activity, and has great research values and a great application prospect.
The efficient synthesis of chiral or achiral tertiary phosphines bearing an o-bromo (or iodo)aryl substituent is described. The key step of this synthesis is based on the reaction of a secondary phosphine borane with the 1,2-dibromo (or diiodo)arene, owing to the formation in situ of an aryne species in the presence of n-butyllithium. When P-chirogenic secondary phosphine boranes were used, the corresponding o-halogeno-arylphosphine boranes were obtained without racemization in moderate to good yields and with ee up to 99%. The stereochemistry of the reaction, with complete retention of the configuration at the P atom, has been established by X-ray structures of P-chirogenic o-halogenophenyl phosphine borane complexes. The decomplexation of the borane was easily achieved without racemization using DABCO to obtain the free o-halogeno-arylphosphines in high yields.
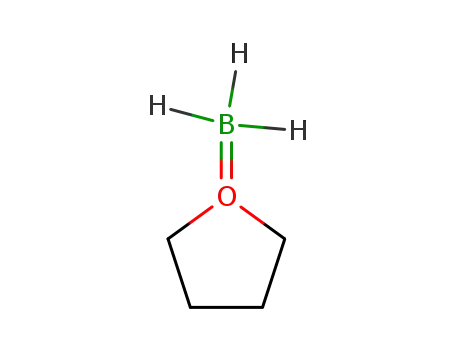
borane-THF

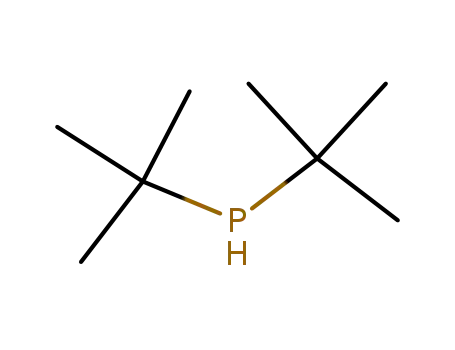
di-tert-butylphosphine

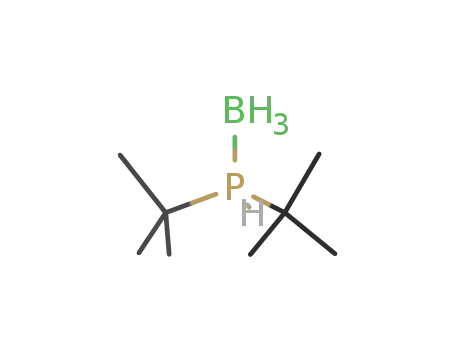
di-tert-butylphosphine borane
| Conditions | Yield |
|---|---|
|
In
tetrahydrofuran;
(N2); BH3*THF in THF added slowly at room temp. to tBu2PH in THF, stirred for 1 h; evapd. (vac.), dissolved in n-pentane, crystd. at 0°C for 7 d;
|
92% |
|
In
tetrahydrofuran;
at 25 ℃;
for 4h;
|
90% |
|
In
tetrahydrofuran;
at 5 - 10 ℃;
for 0.5h;
|
90% |
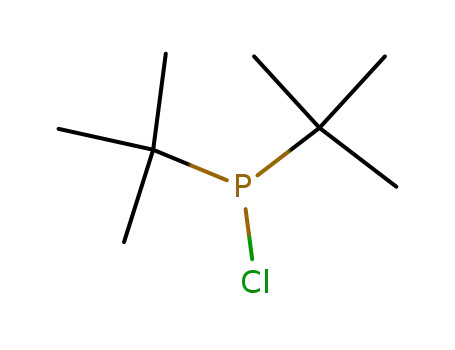
di(tert-butyl)chlorophosphine

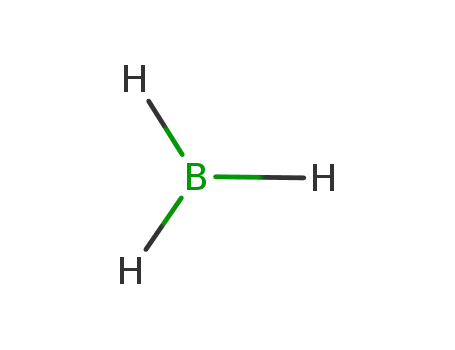
borane

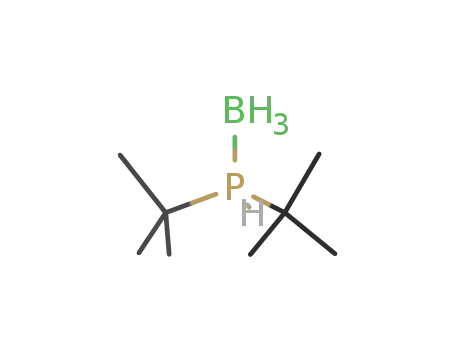
di-tert-butylphosphine borane
| Conditions | Yield |
|---|---|
|
di(tert-butyl)chlorophosphine;
With
lithium aluminium tetrahydride;
In
diethyl ether;
at 20 ℃;
Cooling;
Inert atmosphere;
borane;
In
tetrahydrofuran;
at 0 - 20 ℃;
|
73% |
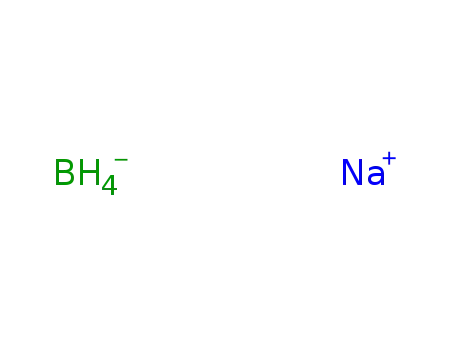
sodium tetrahydroborate

di(tert-butyl)chlorophosphine

borane-THF

di-tert-butylphosphine
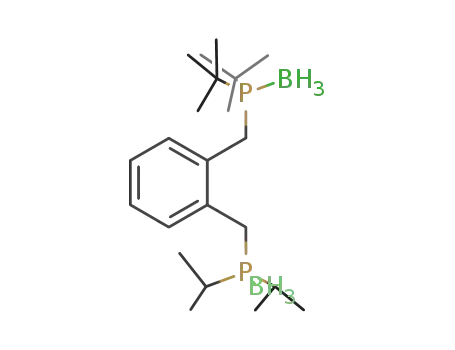
di(tert-butyl)((2-[(diisopropylphosphino)methyl]phenyl)methyl)phosphine-borane (1/2)
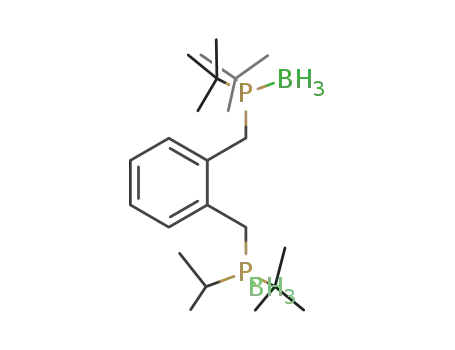
di(tert-butyl)((2-([(tert-butyl)isopropylphosphino]methyl)phenyl)methyl)phosphine-borane (1/2)
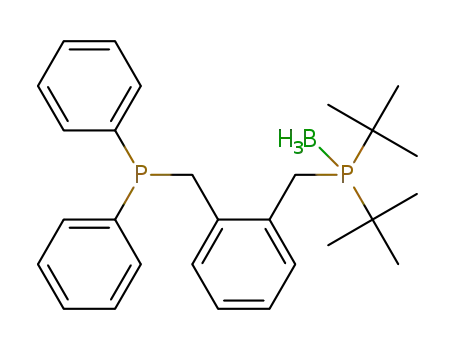
1-(di-tert-butylphosphino{borane}methyl)-2-(diphenylphosphinomethyl)benzene
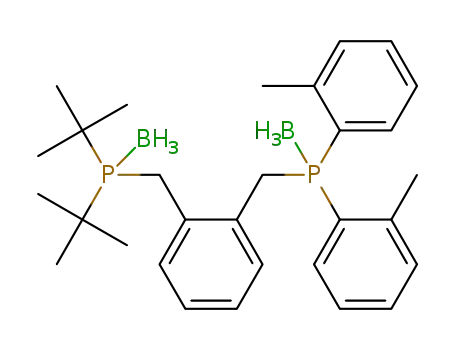
1-(di-tert-butylphosphino{borane}methyl)-2-(di-o-tolylphosphino{borane}methyl)benzene
CAS:610-09-3
Molecular Formula:C<sub>8</sub>H<sub>12</sub>O<sub>4</sub>
Molecular Weight:172.18
CAS:76189-56-5
CAS:554-70-1