Your Location:Home >Products >Organic phosphines >Other organic phosphines >76189-56-5
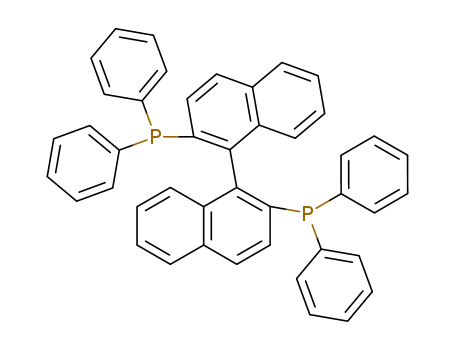

Product Details
Reaction
(R)-BINAP or (R)-Tol-BINAP can be combined with dichloro(1,5-cyclooctadiene)ruthenium to form precursors to NOYORI CATALYST SYSTEMS. These systems exhibit very high catalytic activity and enantioselectivity in the hydrogenation of a wide range of substrates. NOYORI CATALYST SYSTEMS have been shown to effect highly enantioselective hydrogenation of functionalized ketones where the substituents are dialkylamino, hydroxy, siloxy, carbonyl, ester, amide or thioester. Useful ligand in asymmetric Heck processes. Ligand employed in palladium-catalyzed asymmetric arylation of ketones. Ligand employed in rhodium-catalyzed 1,4-additions to enones. Ligand employed in palladium-catalyzed hydroamination of styrene derivatives. Ligand employed in silver-catalyzed asymmetric Sakuri-Hosomi allylation and Mukaiyama aldol reaction. Ligand employed in rhodium-catalyzed kinetic resolution of enynes. Ligand employed in asymmetric rhodium-catalyzed hydroboration of cyclopropenes. Ligand employed in silver-catalyzed a-hydroxylation of stannyl enol ethers. Ligand employed in palladium-catalyzed synthesis of chiral allenes.
Chemical Properties
white to light yellow crystal powde
Uses
Chiral ligand for metal mediated asymmetric catalysis.
Uses
Reactant involved in:Enantioselective and diastereoselective unpoled carbonyl allylationSyntehsis of organophophine oxides as anittumor agentsSN2 halogenation of hydroxy groupsSynthesis of BINAP complexesStudies of conformational flexibility of BINAP chelates
Uses
2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl and its rhodium and ruthenium derivatives are highly selective homogeneous catalysts used for the reduction of aryl ketones, β-keto esters, and α-amino ketones. They have also been used for asymmetric hydrogenation and hydroformylation of olefins, asymmetric Heck reactions, and asymmetric isomerizations of allyls.Ligand used in a palladium-catalyzed, asymmetric, tandem Heck reaction-carbanion capture process leading to a synthesis of a tricyclic sesquiterpene. Also used in a ruthenium-catalyzed asymmetric hydrogenation of α,β-unsaturated acids.
Reduction of phosphine oxides into the corresponding phosphines using PhSiH3 as a reducing agent and Ph3C+[B(C6F5)4]? as an initiator is described. The process is highly efficient, reducing a broad range of secondary and tertiary alkyl and arylphosphines, bearing various functional groups in generally good yields. The reaction is believed to proceed through the generation of a silyl cation, which reaction with the phosphine oxide provides a phosphonium salt, further reduced by the silane to afford the desired phosphine along with siloxanes. (Figure presented.).
Herein reported is the convenient and efficient strategy for the preparation of binaphthyl-based phosphines through direct modification to the commercially available 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP) with sodium. In the absence of 15-crown-5-ether, a cyclic sodium dinapthylphospholide intermediate is mainly generated. With 15-crown-5-ether, P-Ph bonds are selectively cleft by Na to produce binaphthyl-based disodium phosphides. The mechanism of selective formation of sodium dinapthylphospholide or binaphthyl-based disodium phosphides is proposed.
The invention relates to a synthesis method of 2, 2 '-bisdiphenylphosphino-1, 1'-binaphthalene, which is realized by the following steps: step 1, carrying out BUCHERER reaction on 1, 1 '-binaphthyl-2-naphthol to generate 1, 1'-binaphthyl-2, 2 '-diamine; 2, subjecting 1, 1 '-binaphthyl-2, 2'-diamine to a Sandmeyer reaction to generate binaphthyl dibromide; and 3, carrying out a Grignard reaction onthe binaphthyl dibromide and diphenyl phosphine chloride to generate 2, 2 '-bisdiphenylphosphino-1, 1'-binaphthalene (BINAP). Bulk chemical raw materials are used and are low in price and easy to obtain, and the production cost is effectively reduced; the method has the advantages of easily available raw materials, high reaction yield, simple post-treatment, facilitation of industrial amplification, and strong industrial application prospect.
The invention discloses a synthetic method of 2,2'-double diphenyl phosphine-1,1'-dinaphthalene. The method comprises the following steps: adding a lithium metal sheet, a ligand, 2,2'-diethoxy-1,1'-dinaphthalene and an ethers solvent are added in a reaction still, heating the materials to the temperature of 60-140 DEG C, reacting the materials for 6-12 hours, dropping chlorodiphenylphosphine at the temperature of 0 DEG C, heating the material to room temperature, and obtaining the product after the reaction is completed; equivalent water quenching is carried out, a product solution is concentrated and filtered, a filter cake is washed with methanol, and vacuum drying is carried out to obtain the product 2,2'-double diphenyl phosphine-1,1'-dinaphthalene (BINAP). The method has the advantages of high yield, low preparation cost, simple post-treatment, high product purity, and is suitable for process enlargement.
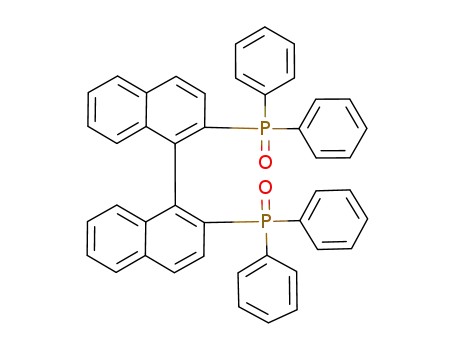
2,2’-bis(diphenylphosphinooxide)-1,1’-binaphthalene

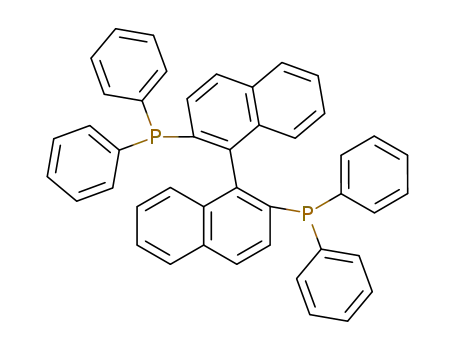
2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl
| Conditions | Yield |
|---|---|
|
With
trichlorosilane; triethyl phosphite;
In
tetrahydrofuran; toluene;
at 100 ℃;
for 3h;
|
95% |
|
With
1,3-diphenyl-disiloxane;
In
toluene;
at 110 ℃;
chemoselective reaction;
Sealed tube;
|
94% |
|
2,2’-bis(diphenylphosphinooxide)-1,1’-binaphthalene;
With
1,1,3,3-Tetramethyldisiloxane;
titanium(IV) isopropylate;
In
toluene;
at 85 ℃;
for 20h;
With
sodium hydroxide; water;
In
toluene;
for 0.25h;
Product distribution / selectivity;
|
91% |
|
2,2’-bis(diphenylphosphinooxide)-1,1’-binaphthalene;
With
trityl tetrakis(pentafluorophenyl)borate;
In
(2)H8-toluene;
at 20 ℃;
Glovebox;
Inert atmosphere;
With
phenylsilane;
In
(2)H8-toluene;
at 100 ℃;
for 24h;
Glovebox;
Inert atmosphere;
Sealed tube;
|
23% |
|
With
[AlH3(triethylamine)];
In
hexane;
at 20 ℃;
for 0.166667h;
Inert atmosphere;
Schlenk technique;
|
96 %Chromat. |
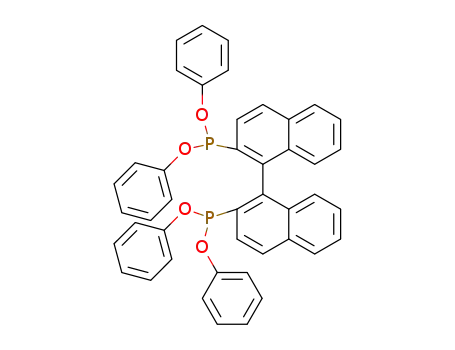
2,2'-bis(diphenyloxyphosphino)-1,1'-binaphthyl


2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl
| Conditions | Yield |
|---|---|
|
With
tributylphosphine; iodine;
In
tetrahydrofuran; acetonitrile;
at 20 ℃;
for 0.166667h;
Inert atmosphere;
|
92% |
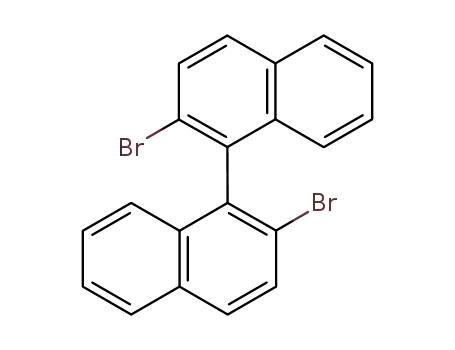
2,2'-dibromo-1,1'-binaphthyl
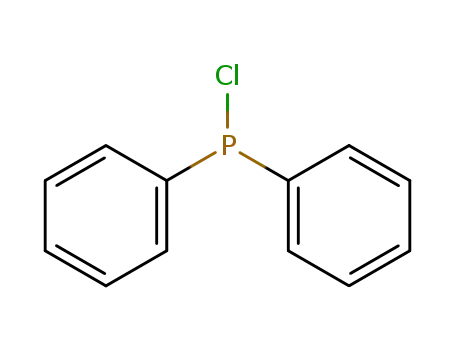
chloro-diphenylphosphine

diphenylarsane
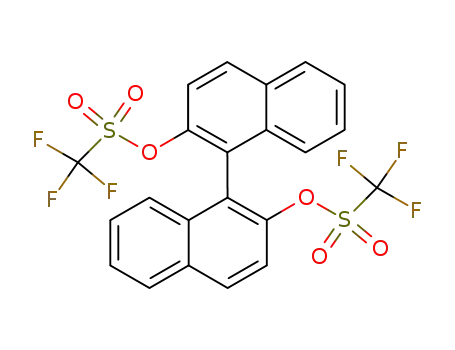
(+/-)-2,2'-bis(trifluoromethanesulfonyloxy)-1,1-binaphthyl
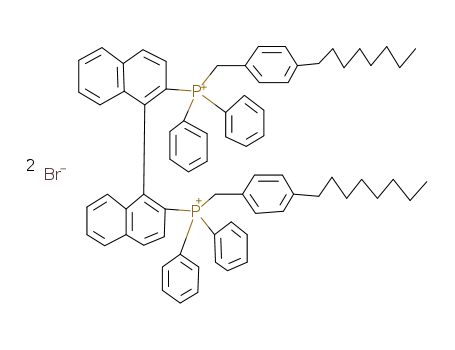
(S)-(+)-1,1'-binaphthyl-2,2'-diylbis<(p-octylbenzyl)diphenylphosphonium> dibromide
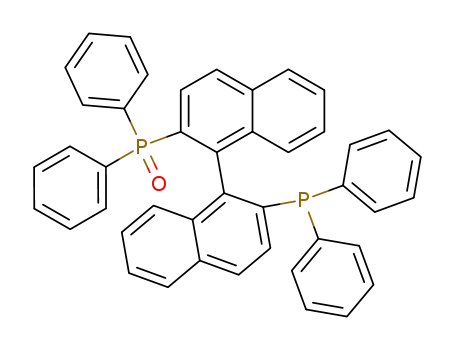
2-(diphenylphosphinyl)-2'-(diphenylphosphanyl)-1,1'-binaphthalene
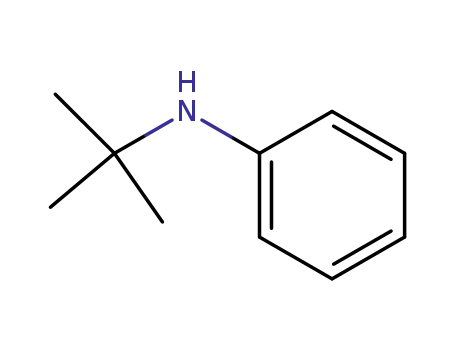
N-tert-butylaniline
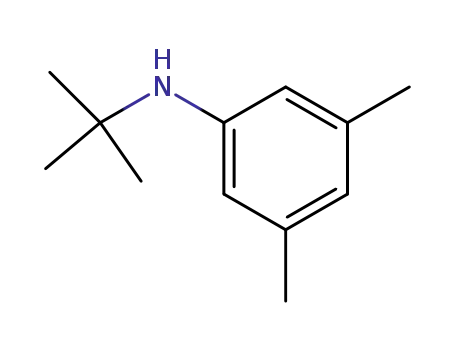
N-(3,5-dimethylphenyl)-tert-butylamine
CAS:1081-34-1
CAS:76189-55-4
Molecular Formula:C44H32P2
Molecular Weight:622.7
CAS:128363-76-8