Your Location:Home >Products >Organic phosphines >Phenyl phosphines >12150-46-8

Product Details
|
Description |
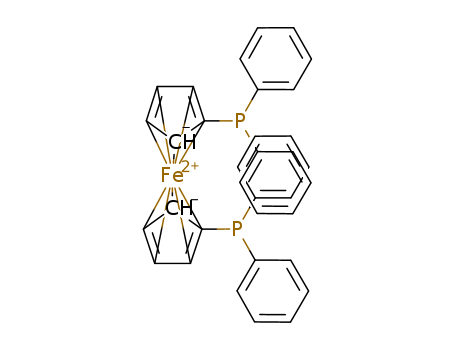
1,1′-Bis(diphenylphosphino)ferrocene (dppf), a diphosphine ligand utilized prominently in homogeneous catalysis, boasts a ferrocene backbone and serves as a pivotal component in various transition metal-catalyzed reactions. Commonly abbreviated as dppf, this compound exhibits deep yellow crystalline properties. |
|
Chemical Properties |
1,1'-Bis(diphenylphosphino)ferrocene is deep yellow crystalline powder |
|
Uses |
1,1'-Bis(diphenylphosphino)ferrocene (dppf) serves as a crucial ligand in homogeneous catalysis, particularly in palladium-catalyzed coupling reactions, but its utility extends to various other transition metals such as rhodium and nickel. It facilitates a broad spectrum of catalytic transformations including cross-coupling reactions, carbon-nitrogen and carbon-oxygen bond formations, Suzuki reactions, hydroaminations, γ-arylation of ketones, and regioselective hydrophosphinylation of terminal alkynes, highlighting its versatility in modern synthetic methodologies. As a stable, redox-active ligand, dppf finds increasing application in coordination chemistry, catalysis, and materials science, underscoring its significance in diverse chemical transformations and materials synthesis. |
(1,1'-Ferrocenediyl)phenyl-phosphine and...
When bisphosphine is taken in a significant excess, the cyclic core rearranges to the linear complex featuring chelating and bridging 1,1′-bis(phosphino)ferrocenes and κ1 coordinated pyrazolate anion. The electronic transitions observed in these complexes are qualitatively described by TD-DFT calculations.
A series of five new heteroleptic 1,1′-bis (diphenylphosphino)ferrocene (dppf) appended nickel (II) dithiolates namely, [Ni (dppf)(benzylcyanidedithiolate)] (Bz-Ni), [Ni (dppf)(2-cyanobenzylcyanidedithiolate)] (CN-Ph-Ni) and [Ni (dppf)(pyridine-2-cyanidedithiolate)] (2-Py-Ni), [Ni (dppf)(pyridine-3-cyanidedithiolate)] (3-Py-Ni), Ni (dppf)(thiophene-2-cyanidedithiolate)] (Th-Ni) have been prepared and characterized using spectroscopic techniques and in one case by single-crystal X-ray diffraction analysis for CN-Ph-Ni.
1,1'-dilithioferrocene
phenylthiodiphenylphosphine
phenyllithium
chloro-diphenylphosphine
3-ethoxypropanal
ethoxypropionaldehyde
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride
palladium
CAS:85-43-8
Molecular Formula:C<sub>8</sub>H<sub>8</sub>O<sub>3</sub>
Molecular Weight:152.15
CAS:19845-69-3
CAS:166330-10-5
Molecular Formula:C36H28OP2
Molecular Weight:538.6