Your Location:Home >Products >OLED intermediates >Carbazoles >1984-49-2
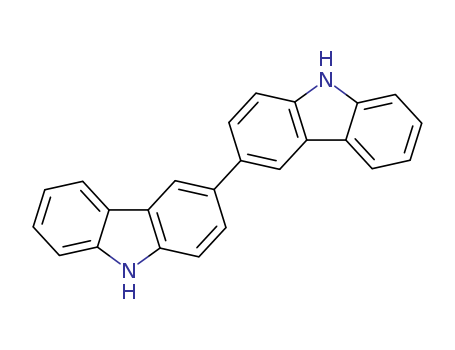

Product Details
Uses
3,3'-bicarbazole can be used to synthesize liquid crystal materials.
Endowing thermally activated delayed fluorescence (TADF) emitter with aggregation-induced emission (AIE) peculiarity is of great significance for realizing more promising commercial applications. Herein, two new dual-emitting-cores emitters with a structu
Low-molar-mass oxiranyl-substituted 3,3'-bicarbazolyl derivatives were synthesized by the reaction of 3,3'-bicarbazolyl-containing oxirane with dimercapto compounds as linking agents. The cationic ring opening polymerization of oxirane monomers was performed using ytterbium (III) trifluoromethanesulfonate [Yb(OTf)3] as a cationic initiator to obtain polyether. The full characterization of the compounds by mass spectrometry, IR, 1H NMR, and 13C NMR is presented. All the compounds represent amorphous materials with glass transition temperatures ranging from 28C to 112C and with 5%-weight-loss temperatures exceeding 308C. The electron photoemission spectra of the materials were recorded, and the ionization potentials of ca. 5.5eV were established. Time-of-flight hole drift mobilities of the amorphous films of some synthesized compounds exceed 10-5cm2/Vs at high electric fields.
A thermally activated delayed fluorescence (TADF) material BCz-2SO has been designed and synthesized as host for blue phosphorescent organic light-emitting diodes (OLEDs). Photophysical studies and theoretical calculations show that the molecule has a small singlet-triplet energy gap (ΔEST) of 0.345 eV, which is beneficial to the reverse energy transferring between the singlet and triplet state. Thanks to the TADF property, the triplet energy can be transmitted to the singlet state through reverse intersystem crossing (RISC), and then transmitted to the guest through the Fo?rster energy transfer (FET) to achieve 100% utilization of energy. Thus, the triplet–triplet annihilation (TTA) of the blue phosphor can be avoided by the extremely low doping concentration of 1%. By using BCz-2SO as the host of FIrpic, the solution-processed blue phosphorescent device achieves the maximum current efficiency (CE), power efficiency (PE), external quantum efficiency (EQE) and highest brightness of 16.38 cd A?1, 9.04 lm W?1, 7.8% and 16,537 cd m-2, respectively. It demonstrates that one can employ the solution-processed method to prepare the high performance phosphorescent OLEDs using the TADF host material we have developed.
Solution-processed organic light-emitting diodes (OLEDs) are attractive due to their low-cost, large area displays, and lighting features. Small molecules as well as polymers can be used as host materials within the solution-processed emitting layer. Herein, we report two 3,3-bicarbazole-based host small molecules, which possess a structural isomer relationship. 9,9-Di-4-n-butylphenyl-9H,9H-3,3-bicarbazole (BCz-nBuPh) and 9,9-di-4-t-butylphenyl-9H,9H-3,3-bicarbazole (BCz-tBuPh) exhibited similar optical properties within solutions but different photoluminescence within films. A solution-processed green phosphorescent OLED with the BCz-tBuPh host exhibited a high maximum current efficiency and power efficiency of 43.1 cd/A and 40.0 lm/W, respectively, compared to the device with the BCz-nBuPh host.
Fabrication of multilayered organic light-emitting diodes (OLEDs) through solution process involves several challenges, especially in preventing dissolution of prior layers during subsequent coating. To overcome, extensive efforts had been made in developing cross-linkable materials. In this work, a thermally cross-linkable hole-transporting material, (9,9′-bis(4-vinylbenzyl)-9H,9′H-3,3′-bicarbazole) (VyPyMCz), is synthesized, characterized and successfully applied to multilayered OLEDs via solution-process. After cross-linking, the hole-transporting material forms robust, smooth and solvent-resistant network, enabling a subsequent spin-coating without deteriorating its film integrity. The measured energy level suggests that VyPyMCz facilitates the injection of hole and effectively blocks electron to realize high efficiency, especially at high luminance. At 1000 cd m?2 for example, the power efficiency of a studied red device is increased from 7.5 to 11.9 lm W?1, an increment of 58%, and the maximum brightness improved from 7724 to 13,560 cd m?2, an increment of 75%, as this electron confining, hole transporting material is incorporated. Remarkably, VyPyMCz also works for a high band gap (2.90 eV) with a high triplet energy (2.80 eV) blue emitter containing OLED device, the power efficiency is increased from 6.6 to 11.8 lm W?1, an increment of 78%, and the maximum luminance enhanced from 5260 to 6857 cd m?2, an increment of 30%, because of its higher triplet energy (2.87 eV).
Oxidative C-C coupling of carbazoles possessing various substituents is demonstrated in the presence of organic (metal-free) recyclable oxidants, such as DDQ or CA/H+, for accessing bicarbazole regioisomers. Differently substituted carbazoles are examined to showcase regioselective discrimination (3,3′-versus 1,3′-bicarbazoles) and preferences based on sterics and electronics in oxidative coupling. Finally, a mechanism that involves the carbazole radical cation has been traced (evidenced) and proposed on the basis of the UV-vis-NIR absorption and EPR spectroscopy results. This study underlines the strategic chemical preparation of a series of bicarbazoles in an efficient manner.
Carbazole analogs 3 and 4 and a new library of bicarbazole-linked triazoles 6–11 were prepared via new synthetic methodology. Metal-catalyzed oxidative coupling reaction was utilized for the synthesis of bicarbazole acetylene 4 and different metals (Znsu
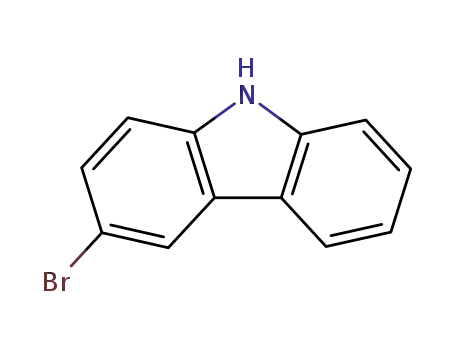
3-bromo-9H-carbazole

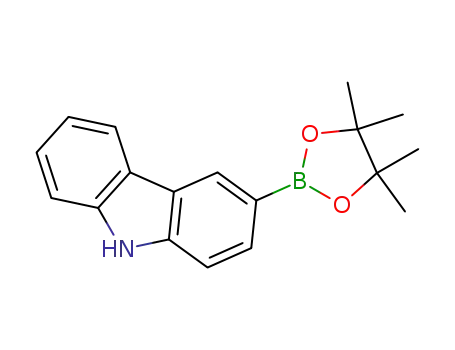
3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)- 9H-carbazole

![9H,9′H-[3,3′]bicarbazole](/upload/2023/2/b9485a18-d8db-4c9d-b2eb-fb302a41a10a.png)
9H,9′H-[3,3′]bicarbazole
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
ethanol; water; toluene;
at 78 ℃;
Inert atmosphere;
|
72.2% |
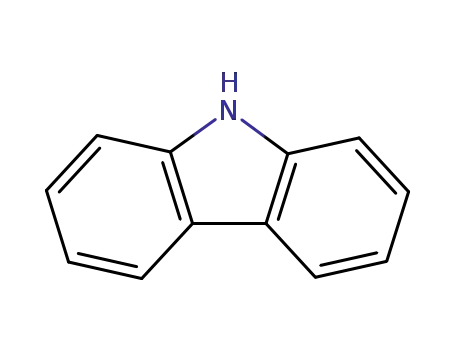
9H-carbazole

![9H,9′H-[3,3′]bicarbazole](/upload/2023/2/b9485a18-d8db-4c9d-b2eb-fb302a41a10a.png)
9H,9′H-[3,3′]bicarbazole
| Conditions | Yield |
|---|---|
|
9H-carbazole;
With
methanesulfonic acid;
In
dichloromethane;
at 0 ℃;
Inert atmosphere;
With
2,3-dicyano-5,6-dichloro-p-benzoquinone;
In
dichloromethane;
at 20 ℃;
for 0.0166667h;
Reagent/catalyst;
regioselective reaction;
Inert atmosphere;
|
93% |
|
With
iron(III) chloride;
In
chloroform;
at 20 ℃;
for 0.5h;
|
84% |
|
With
iron(III) chloride;
In
chloroform;
at 25 ℃;
for 3h;
Inert atmosphere;
|
71% |
|
With
iron(III) chloride;
In
chloroform;
at 20 ℃;
|
62% |
|
With
iron(III) chloride;
In
chloroform;
at 20 ℃;
for 12h;
|
60% |
|
9H-carbazole;
With
iron(III) chloride;
In
chloroform;
at 20 ℃;
for 0.5h;
Inert atmosphere;
With
acetic acid; zinc;
In
methanol; chloroform; ethyl acetate;
at 50 ℃;
Inert atmosphere;
|
22% |
|
With
iron(III) chloride;
In
methanol;
at 20 ℃;
for 22h;
|
11% |
|
With
ammonium vanadate; sulfuric acid;
at 24.9 ℃;
Mechanism;
electrooxidation; var. temperatures and reagents concentrations;
|
|
|
With
sodium dichromate; sulfuric acid; acetic acid;
anschliessend Erwaermen mit NaHSO3-Loesung;
|
|
|
With
iron(III) chloride;
In
chloroform;
|
|
|
With
iron(III) chloride;
In
chloroform;
at 20 ℃;
for 2h;
|
|
|
9H-carbazole;
With
iron(III) chloride;
In
chloroform;
at 20 ℃;
for 22h;
Inert atmosphere;
In
methanol;
for 1h;
In
tetrahydrofuran;
for 0.5h;
Reflux;
|
14 g |
|
With
iron(III) chloride;
In
chloroform;
|

9H-carbazole
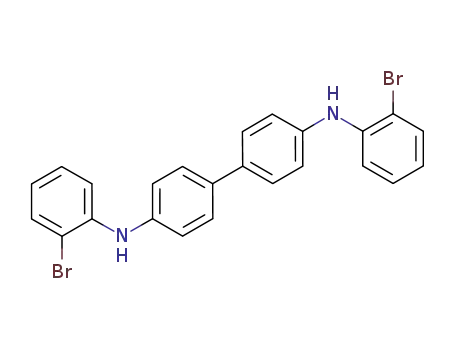
N4,N4'-bis(2-bromophenyl)biphenyl-4,4'-diamine

3-bromo-9H-carbazole
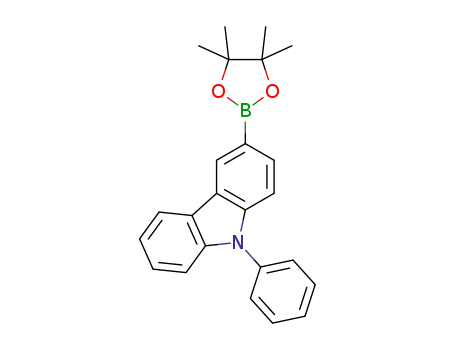
9-phenyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)-9H-carbazole
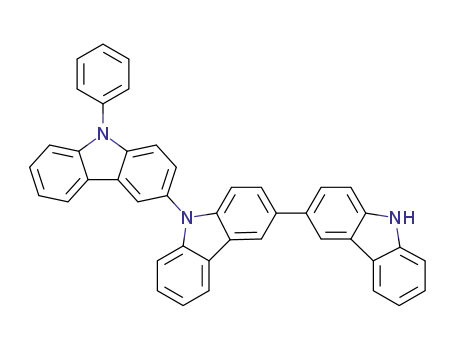
C42H27N3
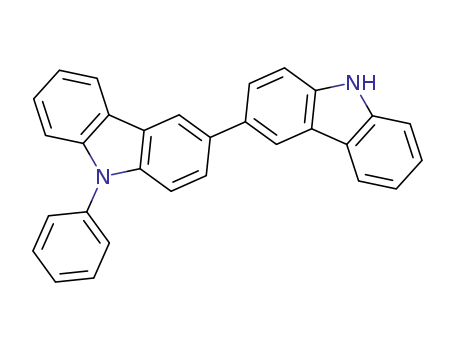
9-phenyl-3,3'-bicarbazole
9-(4,6-diphenyl-1,3,5-triazin-2-yl)-9'-phenyl-3,3'-bicarbazole

C46H30N4
CAS:25550-51-0
Molecular Formula:C<sub>9</sub>H<sub>12</sub>O<sub>3</sub>
Molecular Weight:168.19
CAS:1198395-24-2
Molecular Formula:C27H23N
Molecular Weight:361.5
CAS:444796-09-2