Your Location:Home >Products >Functional intermediates >54446-36-5

Product Details
Chemical Properties
White solid
InChI:InChI=1/C12H10BrN/c13-10-6-8-12(9-7-10)14-11-4-2-1-3-5-11/h1-9,14H
Highly efficient blue emitting material (DAnP) consisting of anthracene and pyrene was designed and synthesized. The PLmax of the DAnP is 469 nm in the solution state and 480 nm in the film state. DAnP was used as non-doped emitting layer (EMLs) in OLEDs with the following structures: ITO/2-TNATA (60 nm)/NPB (15 nm)/DAnP (35 nm)/Alq3 (20 nm)/LiF (1 nm)/Al (200 nm). The DAnP device has current efficiency of 5.45 cd/A, power efficiency of 2.71 lm/W, and CIEs of (0.19, 0.40) at 10 mA/cm2. An efficient multilayer white organic light-emitting diode (WOLED) with the structure of ITO/NPB (30 nm)/CBP: 3 wt% Ir(piq)3 (10 nm)/DAnP (40 nm)/TPBi (40 nm)/LiF (1 nm)/Al (200 nm) was fabricated and characterized, where DAnP and tris(1-phenylisoquinoline) iridium (III) [Ir(piq)3] were used as a blue fluorescent emitter and a red phosphorescent emitter respectively. A WOLED showed current efficiency of 5.08 cd/A, power efficiency of 2.55 lm/W, and CIEs of (0.35, 0.36) at 10 mA/cm2.
Nine novel Schiff bases were derived from salicylic aldehyde and oxalic aldehyde, isolated, and their molecular and spatial structure were explored by a set of experiments (IR, CNMR, HNMR, CHN, SEM, XRD) and theoretical simulation (DFT def2-TZVP). A high potential was predicted in metal cations chelating. The isolated organic species were applied as the ligands in the reaction of complex formation with titanium (III) chloride and (IV) bromide and 12 novel complexes were synthesized and studied experimentally and theoretically. Using the UV–vis spectroscopic titration, the solution stability of the complexes was indicated. Depending on the nature of the Schiff base ligand, their formation constants were calculated in the range of 6.84–17.32. Using the DFT def2-TZVP theoretical method together with the experimental spectroscopic data, the coordination types of the ligands were investigated, and the structure of the complexes was proposed. The photocatalytic ability of the isolated complexes was tested in the C-N cross-coupling reaction under sunlight. Complexes exhibited high visible-light photocatalytic activity for a wide range of aromatic and benzylic amines including electron-withdrawing and electron-donating groups from moderate to good yields ranging in 50–85 %. The use of an inexpensive, clean, and renewable energy source (visible light) is the superiority of the developed photocatalytic systems.
The present study aims to prepare an effective and eco-friendly nanocatalyst for the Chan–Lam coupling reaction of phenylboronic acid and amine in aerobic conditions. For this purpose, chitosan was extracted from shrimp shells waste by demineralization, deproteinization, and deacetylation processes and then converted to chitosan nanoparticles (CSN) by the ionic gelation with tripolyphosphate anions. Afterward, poly-2-hydroxyaniline (P2-HA) was grafted to chitosan nanoparticles (NPs) to employ as the support for CuO NPs. Characterization of the nanocatalyst was done using Fourier transform infrared (FT-IR), X-ray powder diffraction (XRD), scanning electron microscopy (SEM), mapping, energy-dispersive X-ray spectroscopy (EDS), transmission electron microscopy (TEM), and thermogravimetric analysis (TGA). The CuO NPs were identified in the spherical shape with an average size of 17 nm. The prepared nanocatalyst exhibited excellent catalytic performance with a high turnover number (TON) and turnover frequency (TOF) for the Chan–Lam coupling reaction of phenyl boronic acid and amines with different electronic properties. The prepared catalyst could be readily recovered and reused for at least five runs without any noticeable change in structure and catalytic performance. Chitosan (CS) was prepared via demineralization, deproteinization, and deacetylation of shrimp shell and chitosan nanoparticles (CSN) were prepared via ionic gelation process. Polymerization of 2-HA on the CSN surface was done to increase functional groups and create active sites for CuO NPs attachments. CuO NPs-P2-HA-CSN nanocomposite has been shown high efficiently for the Chan–Lam coupling reaction.
Simple copper salts serve as catalysts to effect C-X bond-forming reactions in some of the most utilized transformations in synthesis, including the oxidative coupling of aryl boronic acids and amines. However, these Chan-Lam coupling reactions have historically relied on chemical oxidants that limit their applicability beyond small-scale synthesis. Despite the success of replacing strong chemical oxidants with electrochemistry for a variety of metal-catalyzed processes, electrooxidative reactions with ligandless copper catalysts are plagued by slow electron-transfer kinetics, irreversible copper plating, and competitive substrate oxidation. Herein, we report the implementation of substoichiometric quantities of redox mediators to address limitations to Cu-catalyzed electrosynthesis. Mechanistic studies reveal that mediators serve multiple roles by (i) rapidly oxidizing low-valent Cu intermediates, (ii) stripping Cu metal from the cathode to regenerate the catalyst and reveal the active Pt surface for proton reduction, and (iii) providing anodic overcharge protection to prevent substrate oxidation. This strategy is applied to Chan-Lam coupling of aryl-, heteroaryl-, and alkylamines with arylboronic acids in the absence of chemical oxidants. Couplings under these electrochemical conditions occur with higher yields and shorter reaction times than conventional reactions in air and provide complementary substrate reactivity.
The invention provides a method for synthesizing diarylamine through an N-arylation reaction of arylamine under copper catalysis. The method comprises the following steps: S1, selecting a proper amount of a reaction reagent, a catalyst, a solvent and the like; S2, sequentially adding a reaction reagent, a catalyst, a solvent and the like into a reaction tube with a magnetic bar; S3, selecting a proper amount of AcOH, and adding the AcOH into the reaction tube; S4, heating the reaction tube; S5, performing oil bath treatment; S6, cooling to room temperature, and diluting; S7, extracting by using ethyl acetate; S8, washing the organic layer with saline water; S9, drying on anhydrous Na2SO4; S10, evaporating under vacuum; and S11, purifying the residues into the pure product through silica gel chromatography. A scheme that arylamine and an environmentally-friendly and stable aryl silicon reagent are subjected to an N-arylation reaction under the catalysis of a cheap copper reagent is provided, Cu(OAc)2 is used as a catalyst to react in DMSO in the atmosphere of O2, the conversion reactivity is good, the substrate range is wide, and the method has good tolerance to reaction substrates with various functional groups under mild reaction conditions.
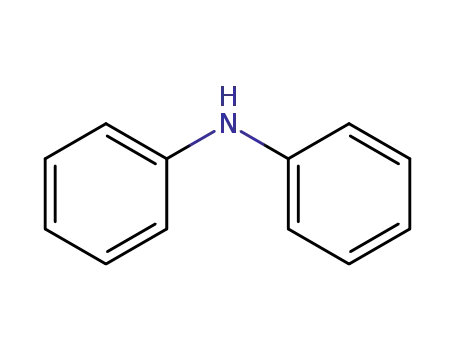
diphenylamine

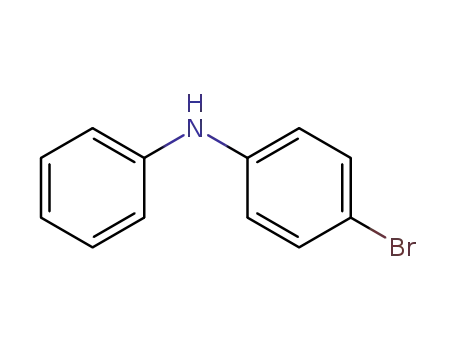
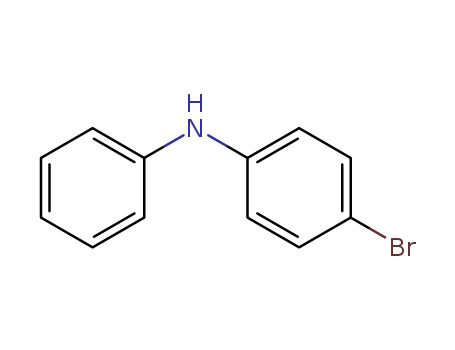
4-bromodiphenylamine
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
ethyl acetate;
for 300h;
|
94% |
|
With
N-Bromosuccinimide;
In
ethyl acetate;
for 30h;
|
94% |
|
With
N-Bromosuccinimide;
In
ethyl acetate;
for 300h;
|
94% |
|
With
N-Bromosuccinimide;
In
ethyl acetate;
for 30h;
|
94% |
|
With
Oxone; copper(ll) bromide;
In
acetonitrile;
at 20 ℃;
for 24h;
regioselective reaction;
|
67% |
|
With
carbon tetrabromide; anthraquinone-2-carboxylic acid;
In
ethanol;
at 20 ℃;
for 70h;
Irradiation;
|
56% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
|
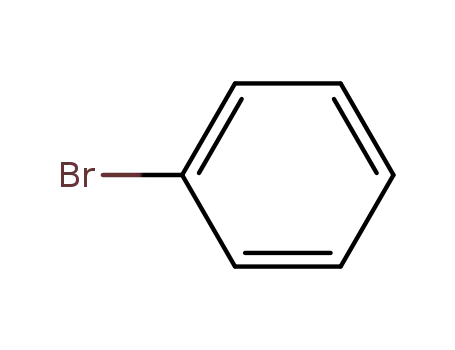
bromobenzene

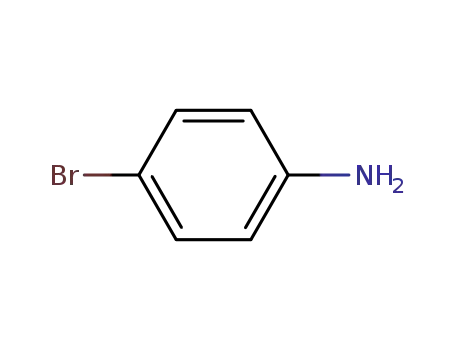
4-bromo-aniline


4-bromodiphenylamine
| Conditions | Yield |
|---|---|
|
With
C27H18N2O4(4-)*2Ti(3+)*2HO(1-); potassium carbonate;
In
N,N-dimethyl-formamide;
at 22 - 34 ℃;
for 6h;
Irradiation;
|
72% |
|
With
CuMoO4; caesium carbonate;
In
dimethyl sulfoxide;
at 90 ℃;
for 21h;
Inert atmosphere;
|
70% |
|
With
tetrabutylammomium bromide; palladium diacetate; potassium carbonate; 2,6-bis(diphenylphosphino)pyridine;
In
N,N-dimethyl acetamide;
at 135 ℃;
for 17h;
Inert atmosphere;
|
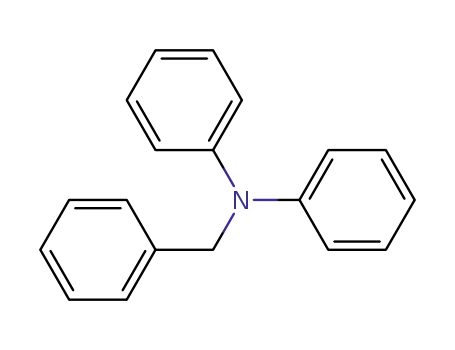
N,N-diphenylbenzylamine
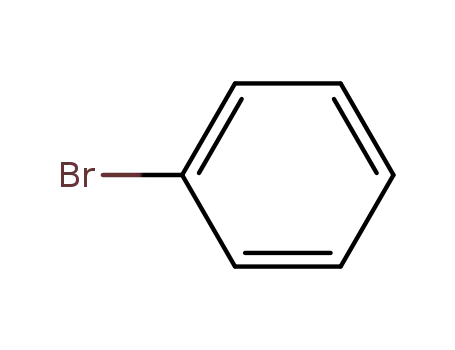
bromobenzene
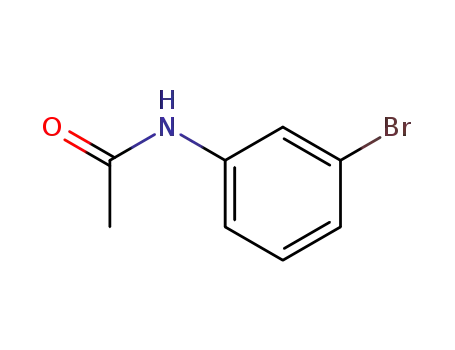
3-bromoacetanilide
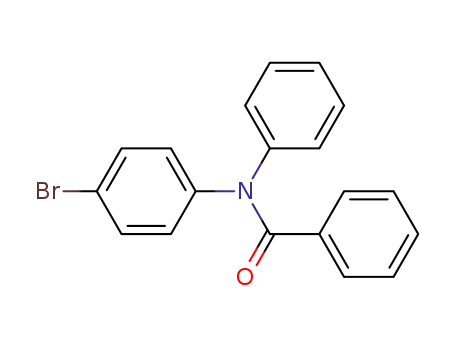
N-(4-bromophenyl)-N-phenylbenzamide
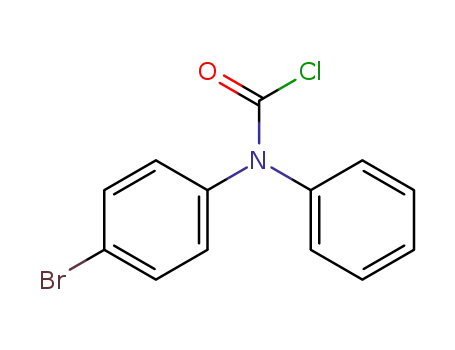
C13H9BrClNO
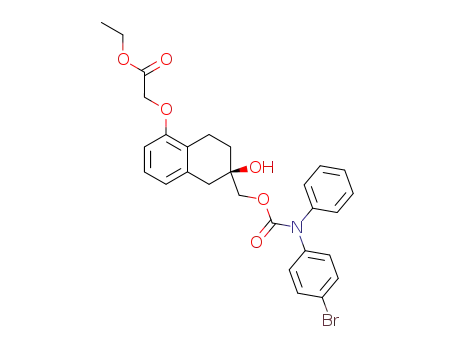
(2R)-2-[(N-4-Bromophenyl-N-phenylcarbamoyloxy)methyl]-5-[(ethoxycarbonyl)methoxy]-2-hydroxy-1,2,3,4-tetrahydronaphthalene
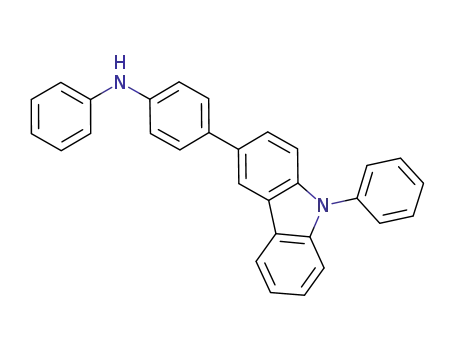
N-phenyl-N-[4-(9-phenylcarbazole-3-yl)phenyl]amine
4-(N-phenyl-N-(4-methoxy)phenylamino)-1-bromobenzene
CAS:571-57-3
CAS:5455-13-0