Your Location:Home >Products >Functional intermediates >571-57-3
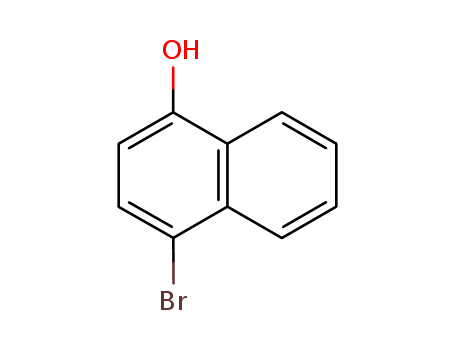

Product Details
Chemical Properties
off-white powder
Synthesis Reference(s)
Canadian Journal of Chemistry, 67, p. 2061, 1989 DOI: 10.1139/v89-320Synthesis, p. 487, 1980 DOI: 10.1055/s-1980-29067
InChI:InChI=1S/C10H7BrO/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6,12H
-
A new procedure for the highly regioselective aerobic bromination of aromatic compounds in the presence of copper-based nanoparticles (CuO/ZnO nanocatalyst) under reflux condition is described. Mechanistic parameters are discussed and the plausible mechanism is proposed. Recyclability of the CuO/ZnO nanocatalyst has also been explored upon aerobic bromination of aromatic compounds.
There is growing interest in creating solids that are responsive to various stimuli. Herein we report the first molecular-level mechanistic picture of the thermochromic polymorphic transition in a series of MAN-NI dyad crystals that turn from orange to yellow upon heating with minimal changes to the microscopic morphology following the transition. Detailed structural analyses revealed that the dyads assemble to create an alternating bilayer type structure, with horizontal alternating alkyl and stacked aromatic layers in both the orange and yellow forms. The observed dynamic behavior in the solid state moves as a yellow wavefront through the orange crystal. The overall process is critically dependent on a complex interplay between the layered structure of the starting crystal, the thermodynamics of the two differently colored forms, and similar densities of the two polymorphs. Upon heating, the orange form alkyl chain layers become disordered, allowing for some lateral diffusion of dyads within their own layer. Moving to either adjacent stack in the same layer allows a dyad to exchange a head-to-head stacking geometry (orange) for a head-to-tail stacking geometry (yellow). This transition is unique in that it involves a nucleation and growth mechanism that converts to a faster cooperative wavefront mechanism during the transition. The fastest moving of the wavefronts have an approximately 38° angle with respect to the long axis of the crystal, corresponding to a nonconventional C-H···O hydrogen bond network of dyad molecules in adjacent stacks that enables a transition with cooperative character to proceed within layers of orange crystals. The orange-to-yellow transition is triggered at a temperature that is very close to the temperature at which the orange and yellow forms exchange as the more stable, while being lower than the melting temperature of the original orange, or final yellow, solids.
The present invention discloses compounds for inhibition of uncontrolled cell proliferation particularly cancer stem cells. Particularly, the invention relates to compounds of Formula I to XXII for the treatment of cancer.
The present disclosure relates generally to certain diacylglycerol lactone compounds, pharmaceutical compositions comprising said compounds, and methods of making and using said compounds and pharmaceutical compositions. The compounds and compositions disclosed herein may be used for the treatment or prevention of diseases, disorders, or infections modifiable by protein kinase C (PKC) agonists, such as HIV.
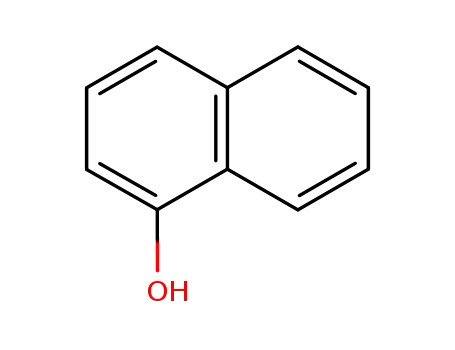
α-naphthol

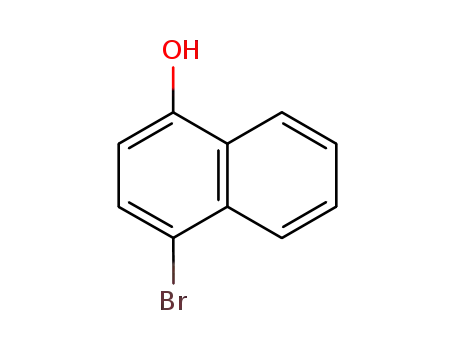
4-bromo-1-naphthol
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 3.5h;
|
96% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 4h;
|
96% |
|
With
tetra-N-butylammonium tribromide;
In
chloroform;
for 0.0333333h;
|
95% |
|
With
tetra-N-butylammonium tribromide;
In
methanol; dichloromethane;
for 0.5h;
Ambient temperature;
reagent 1.0 equivalent;
|
93% |
|
With
N-benzyl-N,N-dimethyl anilinium peroxodisulfate; potassium bromide;
In
acetonitrile;
for 5h;
regioselective reaction;
Reflux;
|
91% |
|
With
benzyltriphenylphosphonium peroxodisulfate; potassium bromide;
In
acetonitrile;
for 5h;
Heating;
|
90% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 4h;
|
90% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 25 ℃;
for 1h;
|
88.3% |
|
With
γ-picolinium bromochromate;
In
acetonitrile;
at 90 ℃;
for 1.33333h;
regioselective reaction;
|
88% |
|
With
potassium bromide;
In
acetonitrile;
for 11h;
regioselective reaction;
Catalytic behavior;
Reflux;
|
87% |
|
With
sodium perborate; potassium bromide;
sodium vanadate;
In
acetic acid;
at 20 ℃;
for 3h;
|
78% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 0 - 20 ℃;
Inert atmosphere;
|
78% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 0 - 20 ℃;
Inert atmosphere;
|
78% |
|
With
trimethylsilyl bromide; bis(4-chlorophenyl)sulfoxide;
In
acetonitrile;
at 0 ℃;
for 6h;
regioselective reaction;
Inert atmosphere;
|
78% |
|
With
N-Bromosuccinimide; boron trifluoride diethyl etherate; tetrabutylammomium bromide;
at 20 ℃;
for 4h;
|
75% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 4.5h;
|
69% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; acetonitrile;
|
64% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 4h;
|
61% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 3h;
|
57% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 1.5h;
|
57% |
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 0 ℃;
for 1h;
|
55% |
|
With
ammonium metavanadate; dihydrogen peroxide; potassium bromide;
In
water; acetonitrile;
for 48h;
Ambient temperature;
pH 5;
|
52% |
|
With
iodine(I) bromide; acetic acid;
|
|
|
With
bromine;
|
|
|
With
bromine; Quinoline sulfate; acetic acid;
|
|
|
With
bromine; iodine;
In
acetic acid;
|
|
|
With
hydrogen bromide; oxygen;
PMoV-2-tetraglyme;
In
1,2-dichloro-ethane;
at 20 ℃;
for 5h;
|
98 % Chromat. |
|
With
1,4-dioxane dibromide;
In
1,4-dioxane;
|
|
|
With
tributylammonium tribromide;
|
|
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 30 ℃;
for 0.05h;
UV-irradiation;
|
|
|
With
N-Bromosuccinimide;
In
acetonitrile;
|
|
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 20 ℃;
for 1.5h;
|
|
|
With
bis(trichloromethyl) carbonate; tetrabutylammomium bromide; potassium bromide;
In
water; ethyl acetate;
at 20 ℃;
for 19h;
regioselective reaction;
|
|
|
With
N-Bromosuccinimide;
In
acetonitrile;
at 25 ℃;
for 16h;
|

α-naphthol


4-bromo-1-naphthol

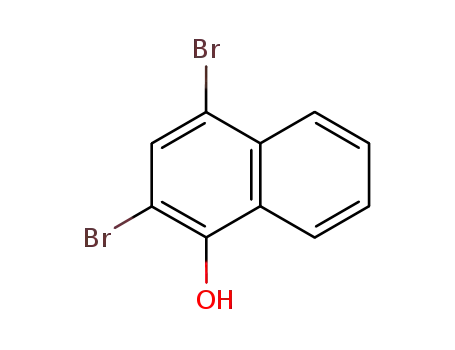
2,4-dibromo-1-naphthol
| Conditions | Yield |
|---|---|
|
With
bis(trichloromethyl) carbonate; tetrabutylammomium bromide; potassium bromide;
In
1,2-dichloro-ethane;
at 40 ℃;
|
99% |
|
With
Diethyl 2-bromomalonate;
at 100 ℃;
for 48h;
Further Variations:;
Reagents;
Product distribution;
|
48% 51% |
t-butyl bromide

α-naphthol
1-bromo-4-methoxynaphthalene
4-hydroxy-1-naphthoic acid
4-bromo-1-ethoxynaphthalene
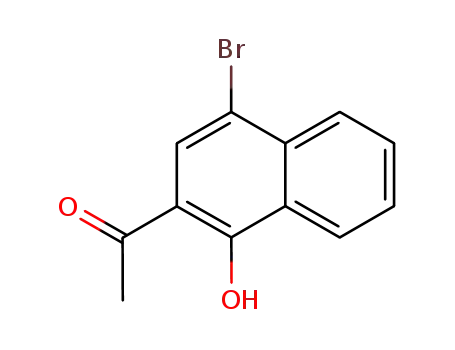
1-(4-bromo-1-hydroxynaphthalen-2-yl)ethanone
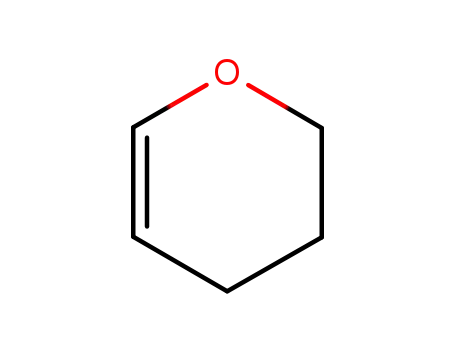
3,4-dihydro-2H-pyran
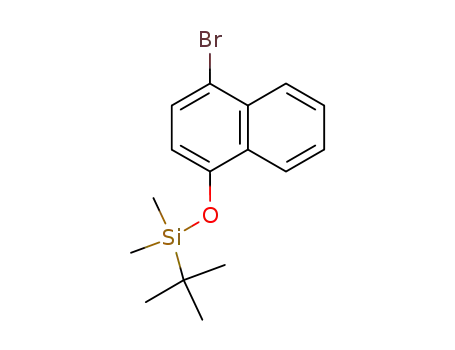
(4-bromonaphthalen-1-yloxy)(tert-butyl)dimethylsilane
CAS:998-40-3
CAS:829-84-5
CAS:65344-26-5
CAS:54446-36-5