Your Location:Home >Products >Functional intermediates >65344-26-5
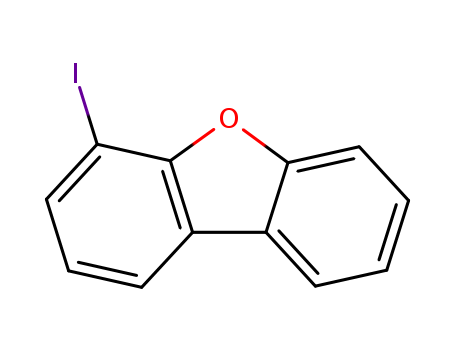

Product Details
-
A benzofuran and acridine merged moiety, 5,5-dimethyl-5,13-dihydrobenzofuro[3,2-c]acridine (BFAc), and a benzothiophene and acridine combined moiety, 5,5-dimethyl-5,13-dihydrobenzothieno[3,2-c]acridine (BTAc), were developed as strong donor moieties of th
The present invention relates to anthracene derivatives which are represented by chemical formula 1 and have high brightness and improved lifespan properties by being included in a light emitting layer, and to an organic electroluminescent device comprising the same.COPYRIGHT KIPO 2017
Xanthone, thioxanthone, fluorenone, benzophenone, 2-benzoylpyridine, dibenzofuran, and dibenzothiophene were deprotonated using a base prepared in situ from MCl 2 ·TMEDA (M = Zn or Cd; TMEDA = N, N, N ′, N ′-tetramethylethylenediamine) and lithium 2,2,6,6-tetramethylpiperidide in a 1:3 ratio, as demonstrated by subsequent iodolysis. The different aryl halides were involved as partners in the N -arylation of pyrrolidin-2-one. In the presence of copper(I) iodide and tripotassium phosphate, and using dimethyl sulfoxide as solvent, the reactions could be performed in yields ranging from 40 to 70%. Most of the products were tested for their antimicrobial, antifungal, antioxidant, and cytotoxic (MCF-7) activity.
The present invention relates to a heterocyclic compound represented by chemical formula A, and an organic light-emitting device containing the same. The ring A, R11-R14, W, X, Y, and Z are the same as defined in the present specification. According to the present invention, the heterocyclic compound exhibits long lifespan and low voltage driving properties when used as a material for phosphorescent hosts.COPYRIGHT KIPO 2017
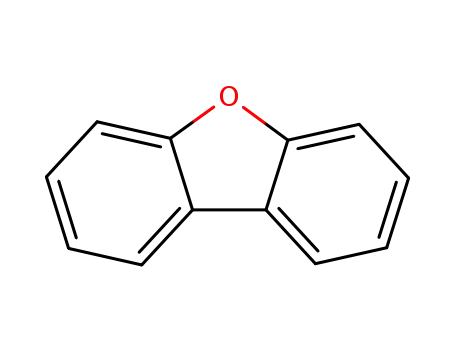
dibenzofuran

![4-Iododibenzo-[b,d]furan](/upload/2023/2/fb71b27f-04ad-45f5-bde3-0dfb00cd8a7d.png)
4-Iododibenzo-[b,d]furan
| Conditions | Yield |
|---|---|
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 4h;
With
iodine;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 2h;
|
91% |
|
dibenzofuran;
With
2,2,6,6-tetramethyl-piperidine; n-butyllithium; zinc dichloro(N,N,N′,N′-tetramethylethylenediamine);
In
tetrahydrofuran; hexane;
at 20 ℃;
for 2h;
Inert atmosphere;
With
iodine;
In
tetrahydrofuran; hexane;
Inert atmosphere;
|
90% |
|
dibenzofuran;
With
n-butyllithium;
In
toluene;
at -78 - 20 ℃;
for 8h;
Inert atmosphere;
With
iodine;
In
tetrahydrofuran; toluene;
at -78 - 20 ℃;
Inert atmosphere;
|
75% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 5h;
With
iodine;
In
tetrahydrofuran;
at -78 - 20 ℃;
|
68.6% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 - 20 ℃;
for 3h;
Inert atmosphere;
With
iodine;
In
tetrahydrofuran;
at 20 ℃;
for 1h;
Inert atmosphere;
|
67% |
|
dibenzofuran;
With
n-butyllithium;
In
tetrahydrofuran;
at -78 ℃;
for 1h;
Inert atmosphere;
With
iodine;
In
tetrahydrofuran;
at 20 ℃;
for 24h;
|
60% |
|
dibenzofuran;
With
n-butyllithium;
In
diethyl ether; hexane;
at -10 - 40 ℃;
for 20h;
With
iodine;
In
diethyl ether; hexane;
at -10 - 20 ℃;
for 3h;
|
48% |
|
dibenzofuran;
With
n-butyllithium;
In
diethyl ether; hexane;
for 16h;
Reflux;
Schlenk technique;
With
iodine;
In
diethyl ether; hexane;
at -8 - 20 ℃;
for 15h;
Schlenk technique;
|
42% |
|
dibenzofuran;
With
n-butyllithium;
In
diethyl ether; hexane;
for 16h;
Inert atmosphere;
Schlenk technique;
Reflux;
With
iodine;
In
diethyl ether; hexane;
at -8 - 20 ℃;
for 15h;
Inert atmosphere;
Schlenk technique;
|
42% |
|
With
n-butyllithium; diethyl ether; iodine;
|
|
|
With
n-butyllithium; iodine;
In
tetrahydrofuran;
|
![2,3-diethynylbenzo[b]furan](/upload/2023/2/77451ba1-1504-4df5-9aea-5d272165a7ad.png)
2,3-diethynylbenzo[b]furan

![4-Iododibenzo-[b,d]furan](/upload/2023/2/fb71b27f-04ad-45f5-bde3-0dfb00cd8a7d.png)
4-Iododibenzo-[b,d]furan
| Conditions | Yield |
|---|---|
|
With
hydrogen iodide; platinum(II) chloride;
In
water;
at 100 ℃;
for 1h;
|
74% |

dibenzofuran
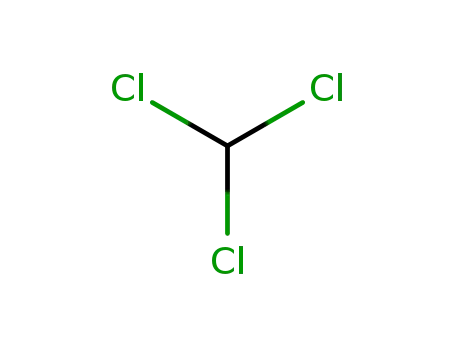
chloroform
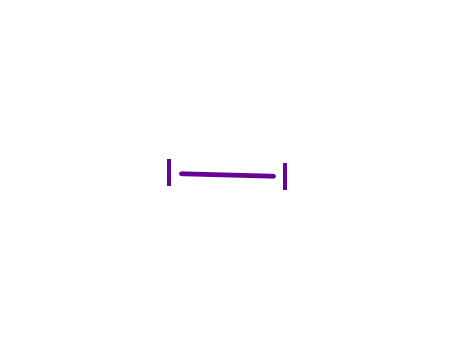
iodine
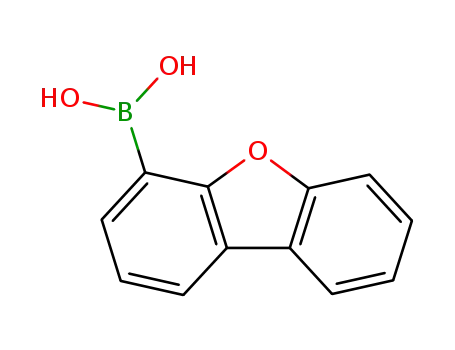
4-dibenzofurylboronic acid
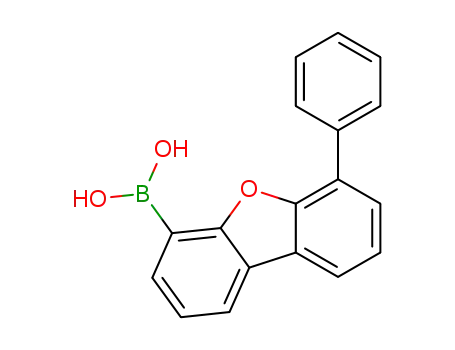
(6-phenyldibenzo[b,d]furan-4-yl)boronic acid
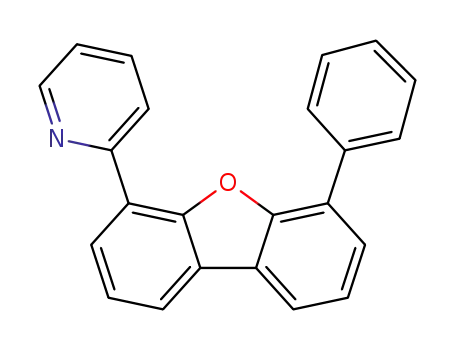
2-(6-phenyldibenzo[b,d]furan-4-yl)pyridine
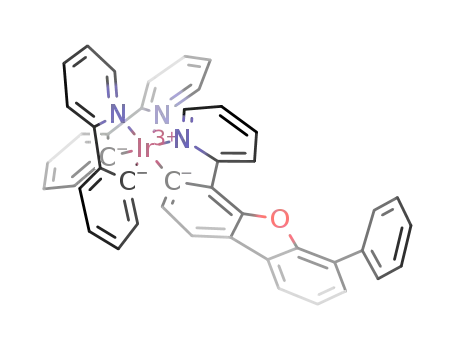
C45H30IrN3O
4-methyl-dibenzofuran
CAS:500717-23-7
Molecular Formula:C30H27N
Molecular Weight:401.5
CAS:85-43-8
Molecular Formula:C<sub>8</sub>H<sub>8</sub>O<sub>3</sub>
Molecular Weight:152.15
CAS:7293-45-0
CAS:571-57-3