Your Location:Home >Products >OLED intermediates >Carbazoles >1153-85-1
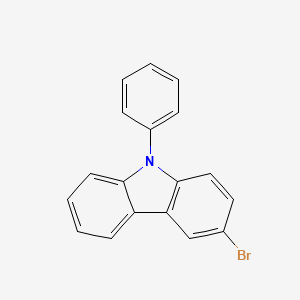

Product Details
Chemical Properties
off-white powder
Uses
Used in manufacturing of OLED materials. Also used as intermediate for pharmaceutical.
Isomeric SMILES: C1=CC=C(C=C1)N2C3=C(C=C(C=C3)Br)C4=CC=CC=C42
InChIKey: KUBSCXXKQGDPPD-UHFFFAOYSA-N
InChI:InChI=1/C18H12BrN/c19-13-10-11-18-16(12-13)15-8-4-5-9-17(15)20(18)14-6-2-1-3-7-14/h1-12H
The electron positive boron atom usually does not contribute to the frontier orbitals for several lower-lying electronic transitions, and thus is ideal to serve as a hub for the spiro linker of light-emitting molecules, such that the electron donor (HOMO)
Briefly, 3-bromo-N-phenylcarbazole was first added into a lithiated THF solution of trimethyl borate and then hydrolyzed in hydrochloric acid to form 9-phenyl-9H-carbazole-3-boronic …
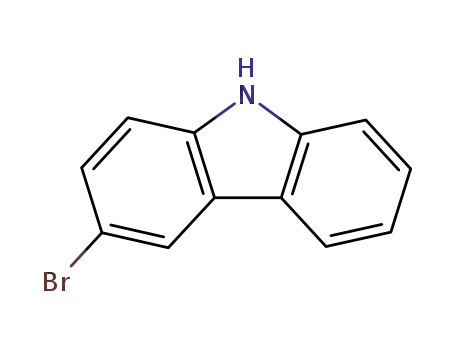
3-bromo-9H-carbazole

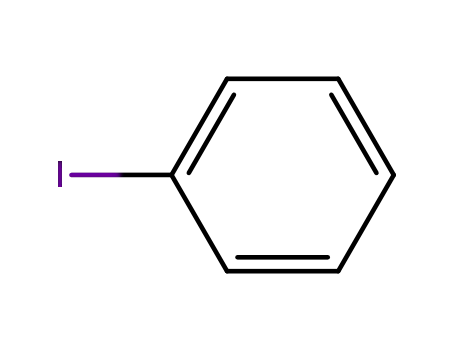
iodobenzene

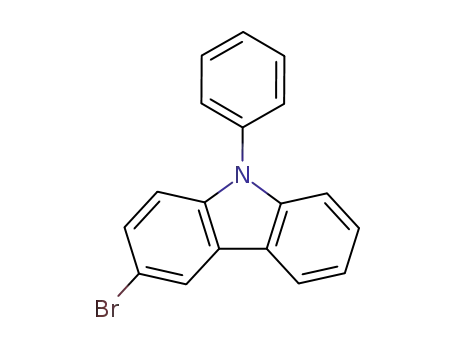
3-bromo-9-phenyl-9H-carbazole
| Conditions | Yield |
|---|---|
|
3-bromo-9H-carbazole; iodobenzene; In N,N-dimethyl-formamide; at 20 ℃; for 0.5h;
With copper(l) iodide; In N,N-dimethyl-formamide; at 100 ℃;
|
93% |
|
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium carbonate; In N,N-dimethyl-formamide; at 100 ℃; for 10h; Inert atmosphere;
|
89.7% |
|
With 18-crown-6 ether; copper; potassium carbonate; In 1,2-dichloro-benzene; at 180 ℃; for 24h;
|
88% |
|
With tris-(dibenzylideneacetone)dipalladium(0); tributylphosphine; In toluene; for 12h; Heating;
|
87.22% |
|
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100 ℃;
|
86% |
|
With copper(l) iodide; 1,10-Phenanthroline; potassium carbonate; In 5,5-dimethyl-1,3-cyclohexadiene; for 6h; Reflux;
|
86% |
|
3-bromo-9H-carbazole; With sodium hydride; In N,N-dimethyl-formamide; for 1h;
iodobenzene; In N,N-dimethyl-formamide;
|
86% |
|
With copper(l) iodide; 1,10-Phenanthroline; 18-crown-6 ether; potassium carbonate; In N,N-dimethyl-formamide; at 150 ℃; for 8h; Inert atmosphere;
|
85.5% |
|
With copper; potassium carbonate; sodium sulfate; In nitrobenzene; at 195 ℃;
|
82% |
|
With copper; potassium carbonate; In nitrobenzene; for 16h; Inert atmosphere; Reflux;
|
81% |
|
With copper; potassium carbonate; In nitrobenzene; for 16h; Inert atmosphere; Reflux;
|
81% |
|
With copper; potassium carbonate; sodium sulfate; In nitrobenzene; at 200 ℃;
|
79% |
|
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100 ℃; for 24h;
|
78% |
|
With potassium carbonate; copper dichloride; In dimethyl sulfoxide; for 12h; Reflux;
|
76% |
|
With tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine; sodium t-butanolate; In toluene; at 100 ℃;
|
75% |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate; In toluene; at 100 ℃;
|
75% |
|
With copper; potassium carbonate; sodium sulfate; In nitrobenzene; at 200 ℃;
|
73% |
|
With copper; potassium carbonate; sodium sulfate; In nitrobenzene; at 200 ℃;
|
72% |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate; In toluene; for 6h; Inert atmosphere; Reflux;
|
65% |
|
With 18-crown-6 ether; copper; sodium t-butanolate; In toluene; at 100 ℃; for 24h;
|
57% |
|
With 18-crown-6 ether; copper; sodium t-butanolate; In toluene; at 100 ℃; for 24h;
|
57% |
|
With copper(I) oxide; 2,2,6,6-tetramethylheptane-3,5-dione; potassium hydroxide; In N,N-dimethyl-formamide; at 130 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
54% |
|
With copper(l) iodide; Inert atmosphere;
|
|
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate; In toluene; at 100 ℃; for 8h;
|
|
|
iodobenzene; With tri-tert-butyl phosphine; palladium diacetate; In toluene; at 20 ℃; for 1h; Inert atmosphere;
3-bromo-9H-carbazole; With potassium carbonate; In toluene; at 80 ℃; for 6h; Inert atmosphere;
|
1.9 g |
|
With copper(l) iodide; Inert atmosphere;
|
|
|
With copper(l) iodide; 1,10-Phenanthroline; potassium hydroxide; In 5,5-dimethyl-1,3-cyclohexadiene;
|
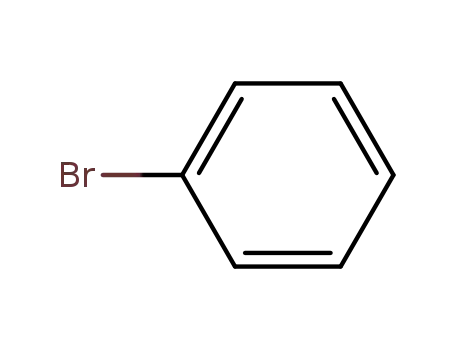
bromobenzene


3-bromo-9H-carbazole


3-bromo-9-phenyl-9H-carbazole
| Conditions | Yield |
|---|---|
|
With dibenzo-18-crown-6; potassium acetate; copper; In N,N-dimethyl-formamide; at 120 ℃; for 4h;
|
71% |
|
With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate; In toluene; at 100 ℃; for 24h;
|
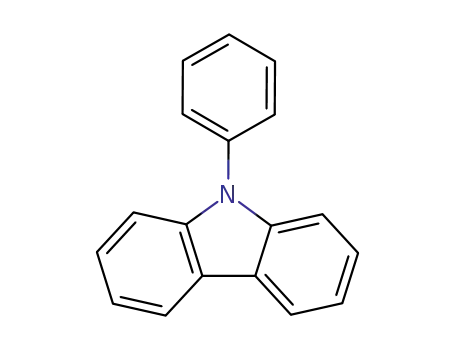
N-phenylcarbazole
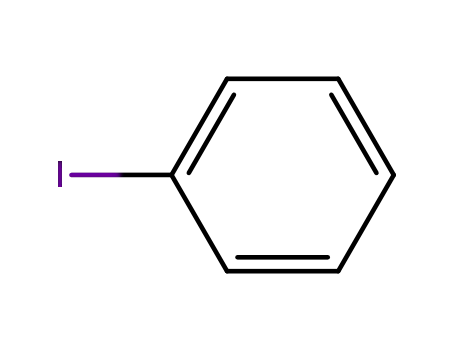
iodobenzene
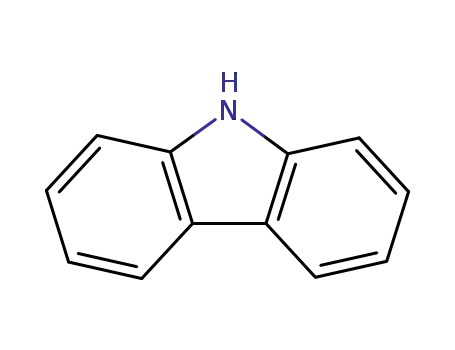
9H-carbazole
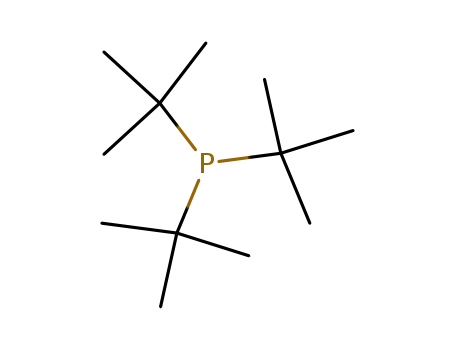
tri-tert-butyl phosphine
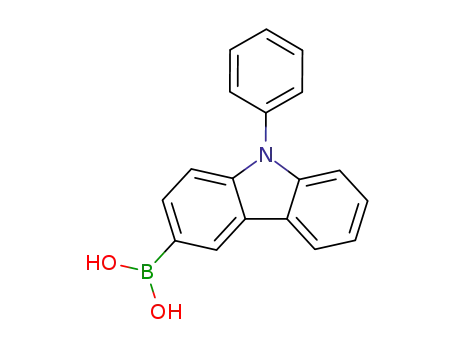
N-phenyl-9H-carbazol-3-boronic acid
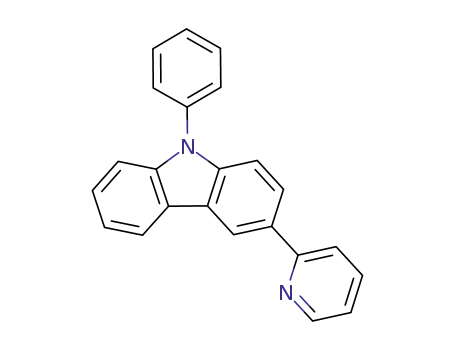
9-phenyl-3-(pyridin-2-yl)-9H-carbazole
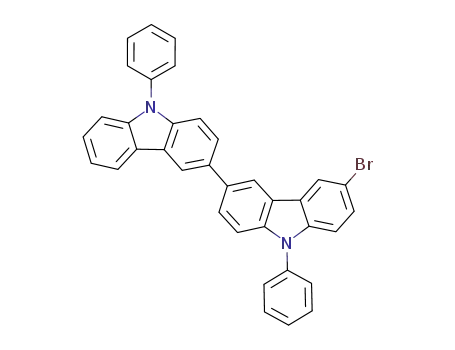
6-bromo-9,9'-diphenyl-9H,9'H-3,3'-bicarbazole
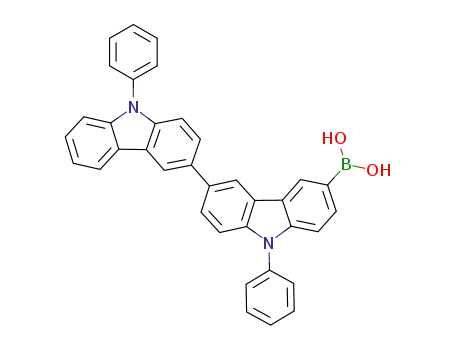
(9,9'-diphenyl-9H,9'H-[3,3'-biscarbazol]-6-yl)boronic acid
CAS:15155-41-6
Molecular Formula:C6H2Br2N2S
Molecular Weight:293.97
CAS:1484-13-5