Your Location:Home >Products >Functional intermediates >1762-84-1


Product Details
Uses
4-Bromo-p-terphenyl is a useful research chemical.
Chemical Properties
White powder
InChI:InChI=1/C18H13Br/c19-18-12-10-17(11-13-18)16-8-6-15(7-9-16)14-4-2-1-3-5-14/h1-13H
Reactions of the [(tppm)Au3Cl3] [tppm = tris(diphenylphosphanyl)methane] precursor and CuI ions with a stoichiometric amount of the corresponding linear mono- [HC2(C 6H4)nC6
Abstract: Possible synthetic routes to 4-bromo-1,1′:4′,1″-terphenyl and 4-methyl-1,1′:4′,1″-terphenyl have been studied. Stevens rearrangement of quaternary ammonium salts containing 3-phenylprop-2-en-1-yl and 3-(4-bromo- or 4-methylphenyl)prop-2-yn-1-yl
PROBLEM TO BE SOLVED: To provide a method for efficiently producing an aromatic compound containing a halogen group(s). SOLUTION: Provided is a method for producing a halogen compound represented by the following general formula (1), comprising reacting an iodine compound represented by the following general formula (2) and a compound represented by the following general formula (3) in the presence of a transition metal compound, at least one phosphine compound selected from the group consisting of 1,1'-bis(diphenylphosphino)ferrocene and 4,5'-bis(diphenylphosphino)-9,9'-dimethylxanthene, and a base. (In the formula, Ar1 and Ar2 each independently represent a C1-40 organic group; X represents a bromine group, a chlorine group, a fluorine group, or a trifluoromethanesulfonate group, and when there are a plurality of X's, they may be the same or different; n represents an integer equal to or larger than 1; R's each independently represent a hydrogen atom, a C1-4 alkyl group, or a phenyl group, and two R's may be linked to form a ring containing oxygen atoms and a boron atom.) SELECTED DRAWING: None COPYRIGHT: (C)2021,JPOandINPIT
Selective C (Formula presented.) –C (Formula presented.) couplings are powerful strategies for the rapid and programmable construction of bi- or multiaryls. To this end, the next frontier of synthetic modularity will likely arise from harnessing the coupling space that is orthogonal to the powerful Pd-catalyzed coupling regime. This report details the realization of this concept and presents the fully selective arylation of aryl germanes (which are inert under Pd0/PdII catalysis) in the presence of the valuable functionalities C?BPin, C?SiMe3, C?I, C?Br, C?Cl, which in turn offer versatile opportunities for diversification. The protocol makes use of visible light activation combined with gold catalysis, which facilitates the selective coupling of C?Ge with aryl diazonium salts. Contrary to previous light-/gold-catalyzed couplings of Ar–N2+, which were specialized in Ar–N2+ scope, we present conditions to efficiently couple electron-rich, electron-poor, heterocyclic and sterically hindered aryl diazonium salts. Our computational data suggest that while electron-poor Ar–N2+ salts are readily activated by gold under blue-light irradiation, there is a competing dissociative deactivation pathway for excited electron-rich Ar–N2+, which requires an alternative photo-redox approach to enable productive couplings.
The invention provides a nitrogen-containing compound represented by a formula I, an electronic element and an electronic device, and belongs to the technical field of organic materials. The nitrogen-containing compound can improve the performance of an electronic component.
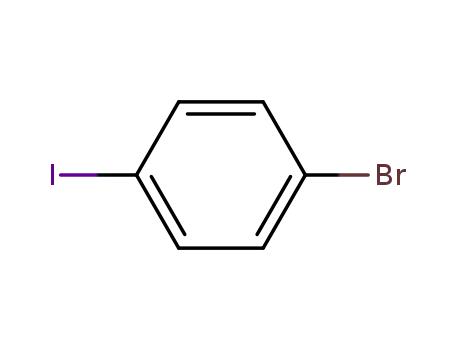
1,4-bromoiodobenzene

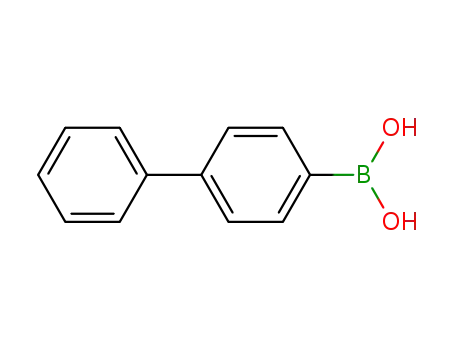
4-biphenylboronic acid


4-bromo-p-terphenyl
| Conditions | Yield |
|---|---|
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran;
at 60 ℃;
for 3h;
|
95% |
|
With
potassium carbonate;
In
tetrahydrofuran; water;
at 60 ℃;
for 3h;
|
95% |
|
With
tetrakis(triphenylphosphine) palladium(0); potassium carbonate;
In
tetrahydrofuran;
at 80 ℃;
for 6h;
Inert atmosphere;
|
90% |
|
1,4-bromoiodobenzene;
With
tetrakis(triphenylphosphine) palladium(0);
In
1,2-dimethoxyethane;
at 20 ℃;
for 0.166667h;
4-biphenylboronic acid;
With
sodium carbonate;
In
1,2-dimethoxyethane;
for 12h;
Further stages.;
Heating;
|
80% |
|
With
potassium carbonate;
tetrakis(triphenylphosphine) palladium(0);
In
ethanol; toluene;
at 100 ℃;
for 5h;
|
74% |
![4'-bromo-[1,1'-biphenyl]-4-yl trifluoromethanesulfonate](/upload/2023/2/7f6f23be-1b35-4d88-807b-2b82086b1295.png)
4'-bromo-[1,1'-biphenyl]-4-yl trifluoromethanesulfonate

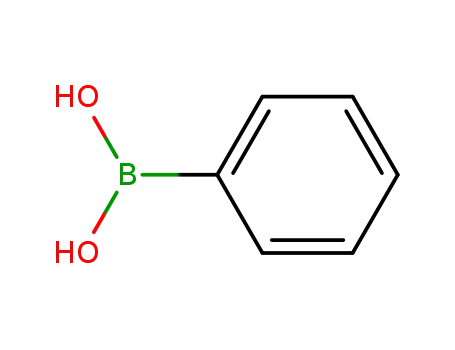
phenylboronic acid

C19H13F3O3S

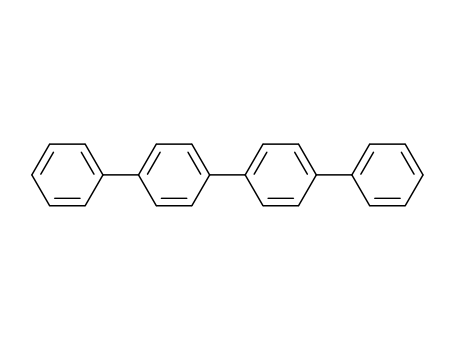
p-quaterphenyl


4-bromo-p-terphenyl
| Conditions | Yield |
|---|---|
|
With
tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; 1,4-di(diphenylphosphino)-butane;
In
water; toluene;
for 16h;
Inert atmosphere;
Heating;
|
31.8% 29% 18.4% |
bromobenzene
N-biphenyl-4-yl-N-nitroso-acetamide
[1,1';4',1'']terphenyl
benzene
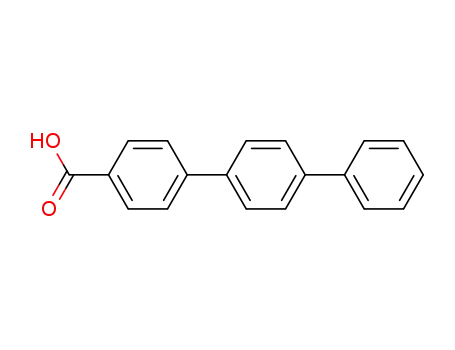
[1,1':4',1''-terphenyl]-4-carboxylic acid
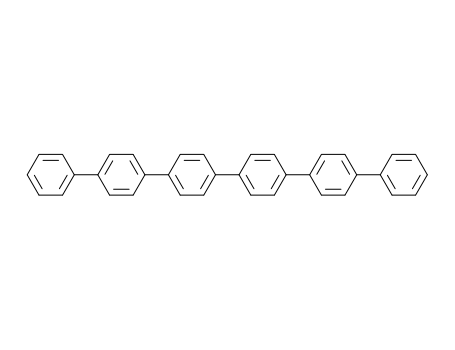
para-hexaphenyl
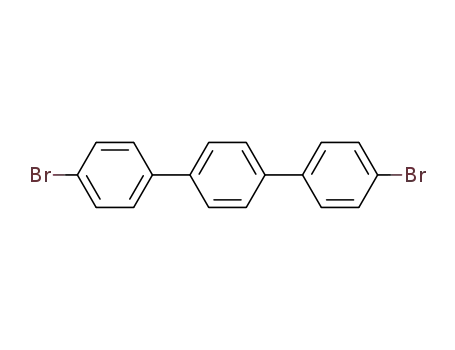
4,4''-dibromo-p-terphenyl
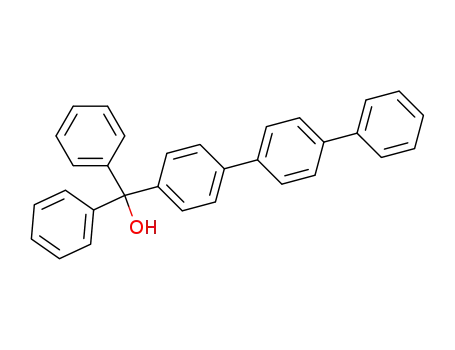
Diphenyl-p-terphenyl-4-yl-methanol
CAS:867374-53-6
CAS:604-53-5
CAS:5455-13-0
CAS:1770840-43-1
Molecular Formula:C6H5IN4
Molecular Weight:260.04