Your Location:Home >Products >OLED intermediates >Fluorenes >1246562-40-2
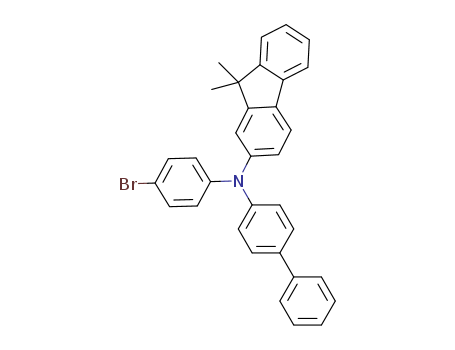

Product Details
Uses
N-([1,1''-Biphenyl]-4-yl)-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine is used as a reactant in the synthesis of novel hole transport materials for efficient green electroluminescent devices.
Three novel triarylamine dyes (AFL1–AFL3) containing fluorenyl and the biphenyl moieties have been designed and synthesized for application in dye-sensitized solar cells. The light-harvesting capabilities and photovoltaic performance of these dyes were investigated systematically through comparison of different π-bridges. The dye with a furan linker exhibited a higher open-circuit voltage (VOC) and monochromatic incident photon-to-current conversion efficiency (IPCE) compared to thiophene and benzene linker. Thus, AFL3 containing a furan linker exhibited the maximum overall conversion efficiency of 5.81% (VOC?=?760?mV,?JSC?=?11.36?mA?cm?2and ff?=?0.68) under standard global AM 1.5 G solar condition.
Disclosed are a heterocyclic compound represented by chemical formula 1 and an organic light emitting device including the same. For details of substituents of chemical formula 1, refer to the detailed description. Provided is an organic light emitting device, which comprises: a first electrode; a second electrode; and an organic layer including a light emitting layer interposed between the first electrode and the second electrode. The organic layer includes two hole transport layers, and one hole transport layer of the hole transport layers includes a compound represented by chemical formula 1.
An organic electroluminescent material and a device thereof are disclosed. The organic electroluminescent material is a monoamine aromatic compound containing a dibenzo selenophen structure. The compound can be used as an organic layer material such as a hole transport material and an electron blocking material in an organic electroluminescent device. By using the compound, the characteristics ofheat resistance, driving voltage, luminous efficiency, service lifetime and the like of the OLED device can be effectively improved.
The invention provides a compound shown as a general formula (I), which can be used for a hole transport material. The compound has a parent structure of difluorene substituted arylamine, is high in bond energy between atoms, has good thermal stability, is beneficial to intermolecular solid accumulation, is strong in hole transition capability, and can effectively reduce device voltage and prolongthe service life of the material when being used as a hole transport material. The invention further provides an organic light-emitting device and a display device containing the compound shown in the general formula (I).
The present invention relates to an organic electroluminescent compound applied to a hole transfer material, which is characterized by being presented by chemical formula 1-1 and chemical formula 1-2. The organic electroluminescent compound according to the present invention can manufacture an organic electroluminescent device having improved light emitting efficiency and life properties, when applied to a hole transfer layer of the organic electroluminescent device.
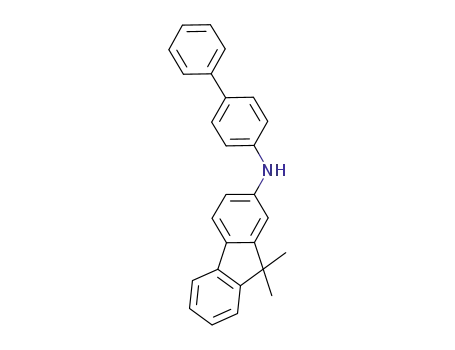
N?(biphenyl?4?yl)?9,9?dimethyl?9H?fluorene?2?amine

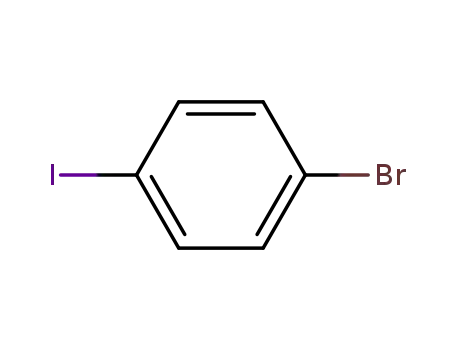
1,4-bromoiodobenzene

![biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)phenylamine](/upload/2023/2/105ed449-2075-471a-a2be-8114630293ca.png)
biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)phenylamine
| Conditions | Yield |
|---|---|
|
With
copper(l) iodide; 1,10-Phenanthroline; potassium hydroxide;
In
o-xylene; water;
at 150 ℃;
for 8h;
Inert atmosphere;
|
91.8% |
|
With
palladium diacetate; sodium t-butanolate;
In
toluene;
Inert atmosphere;
Reflux;
|
78% |
|
N?(biphenyl?4?yl)?9,9?dimethyl?9H?fluorene?2?amine; 1,4-bromoiodobenzene;
In
1,4-dioxane;
for 1h;
Inert atmosphere;
With
sodium t-butanolate;
tri-tert-butyl phosphine; palladium diacetate;
In
1,4-dioxane;
for 18h;
Reflux;
|
70% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; potassium tert-butylate;
In
toluene;
at 85 ℃;
for 4h;
|
69% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; potassium tert-butylate;
In
toluene;
at 85 ℃;
for 4h;
|
69% |
|
With
sodium t-butanolate;
tris-(dibenzylideneacetone)dipalladium(0); triphenylphosphine;
In
toluene;
at 130 ℃;
for 24h;
|
68% |
|
With
dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 18h;
Inert atmosphere;
|
64% |
|
With
dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate; sodium t-butanolate;
In
toluene;
at 90 ℃;
for 18h;
Inert atmosphere;
|
64% |
|
1,10-Phenanthroline; copper(l) chloride;
In
toluene;
at 180 ℃;
for 2h;
Inert atmosphere;
|
58% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate;
In
toluene;
at 50 ℃;
|
47% |
|
With
bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate;
In
5,5-dimethyl-1,3-cyclohexadiene;
at 120 ℃;
for 12h;
|

N-(1,1‘-biphenyl-4-yl)-9,9-dimethyl-N-phenyl-9H-fluoren-2-amine

![biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)phenylamine](/upload/2023/2/105ed449-2075-471a-a2be-8114630293ca.png)
biphenyl-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)phenylamine
| Conditions | Yield |
|---|---|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 12.5h;
|
90% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; toluene;
at 20 ℃;
for 2.5h;
|
89% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; toluene;
at 20 ℃;
for 2.5h;
|
89% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; toluene;
at 20 ℃;
for 2.5h;
|
89% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; toluene;
at 20 ℃;
for 2.5h;
|
89% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; toluene;
at 20 ℃;
for 2.5h;
|
89% |
|
With
N-Bromosuccinimide;
In
ethyl acetate; toluene;
at 20 ℃;
for 2.5h;
|
89% |
|
With
N-Bromosuccinimide;
In
chloroform;
for 4h;
|
74.9% |
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 12.5h;
|
|
|
With
N-Bromosuccinimide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 6.5h;
|
6.4 g |

N?(biphenyl?4?yl)?9,9?dimethyl?9H?fluorene?2?amine
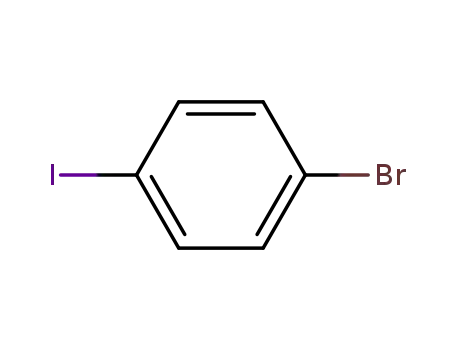
1,4-bromoiodobenzene
bromobenzene
1.4-dibromobenzene
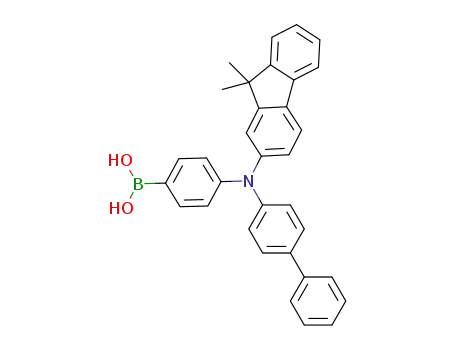
[4-[[1,1’-biphenyl]-4-yl-(9,9-dimethyl-9H-fluoren-2-yl)amino]phenyl]boronic acid

N-(1,1‘-biphenyl-4-yl)-N-[4-(9-phenyl-9H-carbazol-3-yl)phenyl]-9,9-dimethyl-9H-fluoren-2-amine
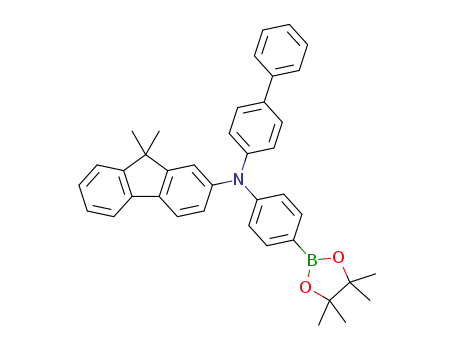
(N-[1,1‘-biphenyl]-4-yl)-9,9-dimethyl-N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)phenyl)-9H-fluorene-2-amine
CAS:1606981-69-4
CAS:145543-83-5
CAS:198964-46-4