Your Location:Home >Products >OLED intermediates >Fluorenes >198964-46-4
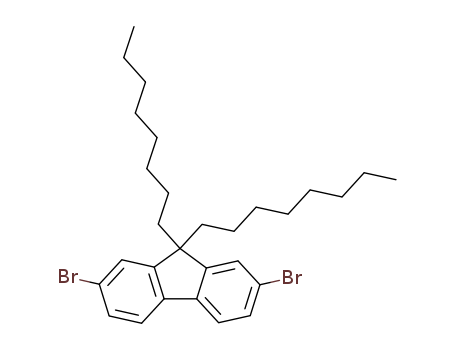

Product Details
Description
9,9-Dioctyl-2,7-dibromofluorene can be used for the synthesis of polymer semiconductors, engaging either Suzuki coupling or Stille coupling reactions.
Chemical Properties
White solid
Uses
9,9-Dioctyl-2,7-dibromofluorene is a reactant in the synthesis of 5,5''-(9,9-dioctyl-9H-fluorene-2,7-diyl)bis(thiophene-2-carbaldehyde), a donor-acceptor type photoelectrical material used in organic light-emitting diodes (OLEDs), organic photovoltaic (OPVs) and organic field-effect transistors (OFETs).
General Description
Intermediate for polymeric light-emitting diodes.
InChI:InChI=1/C29H40Br2/c1-3-5-7-9-11-13-19-29(20-14-12-10-8-6-4-2)27-21-23(30)15-17-25(27)26-18-16-24(31)22-28(26)29/h15-18,21-22H,3-14,19-20H2,1-2H3
Octaoctyl substituted fluorenophanetetraene has been synthesized from its corresponding octaoctyl substituted tetrathia[3.3.3.3]fluorenophane followed by benzyne Stevens rearrangement, oxidation and thermal elimination. The solid-state structure of octaoctyl substituted [2.2.2.2](2,7)-fluorenophanetetraene reveals that fluorene units are connected by cis vinylenes and alkyl chains are located inside and outside the rings. The photooxidation reaction of the fluorenophanetetraene was carried out in a dilute solution under UV light irradiation to give all trans linear fluorenevinylene with aldehyde end groups. The optical properties of the fluorenophanetetraene and its linear compound generated by photooxidation have been investigated and compared. The fluorescence quantum yield of the fluorenophanetetraene in solution is much lower than that of its linear compound generated by photooxidation due to shorter effective conjugation lengths; however, the fluorescence intensity of the fluorenophanetetraene in the solid state is much higher than that of its linear compound generated by photooxidation possibly due to weak intermolecular interaction.
In the solid state, the title compound, C29H40Br2.-0.25CHCl3, crystallizes in the tetragonal system. The fluorene ring system is perfectly planar. The torsion angles in the octyl chains are all trans. These octyl chains are all orthogonal to the aromatic ring and are in the ab plane of the unit cell. The stacking of aromatic units is non-existent. In the cyclopentadiene ring, the angle at the benzilic carbon is 101.1 (3)° and is smaller than other angles in this part of the fluorene ring system.
The synthesis of fluorene-based π-conjugated polymers via sequential bromination and Pd-catalyzed direct arylation polycondensation was demonstrated; the protocol allows dual functionalization of each aromatic monomer in one-pot fashion. This synthetic protocol afforded the corresponding polymers with high molecular weights in good yields (up to yield 80%, Mn 34,500).
(Chemical Equation Presented) Copolymers of phosphafluorenes are obtained through Suzuki copolymerization. The phosphorus-containing copolymers show unique optical, electrochemical, and optoelectronic properties. Blue and white electroluminescence can be
A novel arylamine based hole transporting material (HTM) with tetraethylene glycol (TEG) side groups is reported. Lithium ions solubilised by the TEG groups are employed to modulate interfacial electron transfer reactions at a dye sensitised TiO2/su
A series of new molecular materials with varying carbazole and fluorene contents were prepared employing Suzuki coupling and their properties were compared. A variation of the core nucleus from fluorene to carbazole was a key point to study its effect on the thermal, optical and electrochemical properties. The materials were characterized by FTIR, 1H-, 13C-NMR, APCI-MS and elemental microanalyses. The synthesized materials exhibit slightly bathochromic shifted UV/Vis spectra in dilute solution and emission maxima in the blue region along with good thermal stability. The dyes with a fluorene core are electrochemically and spectrally stable with high lying HOMO levels, in contrast to the carbazole only materials, having potential as light emitting and hole transporting materials in organic light emitting diodes.
We report a new route for the design of electroluminescent polymers by grafting high-efficiency phosphorescent organometallic complexes as dopants and charge transport moieties onto alky side chains of fully conjugated polymers for polymer light-emitting diodes (PLED) with single layer/single polymers. The polymer system studied involves polyfluorene (PF) as the base conjugated polymer, carbazole (Cz) as the charge transport moiety and a source for green emission by forming an electroplex with the PF main chain, and cyclometalated iridium (Ir) complexes as the phosphorescent dopant. Energy transfer from the green Ir complex or an electroplex formed between the fluorene main chain and side-chain carbazole moieties, in addition to that from the PF main chain, to the red Ir complex can significantly enhance the device performance, and a red light-emitting device with the high efficiency 2.8 cd/A at 7 V and 65 cd/m2, comparable to that of the same Ir complex-based OLED, and a broad-band light-emitting device containing blue, green, and red peaks (2.16 cd/A at 9 V) are obtained. Copyright
Two C60 dumbbell molecules have been synthesized containing either cyclopropane or pyrrolidine rings connecting two fullerenes to a central fluorene core. A combination of spectroscopic techniques reveals that the cyclopropane dumbbell possesses better electronic communication between the fullerenes and the fluorene. This observation is underpinned by DFT transport calculations, which show that the cyclopropane dumbbell gives a higher calculated single-molecule conductance, a result of an energetically lower-lying LUMO level that extends deeper into the backbone. This strengthens the idea that cyclopropane behaves as a quasi-double bond.
Five novel small molecules were designed and synthesized to investigate the relationships between molecular structures and photoelectric properties. Firstly, three A1-A-A1 type molecules, incorporating a 2,1,3-benzothiadiazole (BT) unit with no, one or two fluorine atoms as the core, 2-ethylhexyl-substituted phthalimide (PI) as the terminal moiety and ethynyl functionalized thiophene as the π-linker, were synthesized to explore the effect of fluorination on photoelectric properties, namely (PIAT)2BT, (PIAT)2fBT and (PIAT)2dfBT. By introducing fluorine atoms on the molecular backbone, both the HOMO and LUMO energy levels can be tuned efficiently, with minimal influence on optical absorption. Next, on the basis of the BT-based molecules, two extended molecules containing diketopyrrolopyrrole (DPP) were constructed, namely (PIAT)2DPP and FADPPPI, improving optical absorption successfully, especially for FADPPPI with an absorption edge of 850 nm in the film. Considering the photoelectric properties of the above five molecules, poly(3-hexylthiophene) (P3HT) was chosen as the electron donor material to pair with these molecules, and preliminary device fabrication and investigation of photovoltaic performances were performed. These results demonstrate that our materials show great potential in being small-molecule acceptors for organic solar cells (OSCs), and further research on device fabrication and optimization is in progress in our laboratory.
Three new dipolar organic dyes of the type D-D-π-A were constructed on fluorenothiophene backbone by varying the terminal donor auxiliary such as -NMe2, -NEt2 and -NPh2. The effect of such structural variation on the photophysical and electrochemical properties has been investigated. Computational studies obtained from DFT/TD-DFT calculations were found to be in good correlation with the experimental optoelectronic results. TiO2 based n-type quasi-solid state DSSCs constructed on the synthesized dyes revealed a gradual increase in photovoltaic performance with increase in donor size. The trend in power conversion efficiency (η) was found to be -NPh2 (4.10%) > -NEt2 (3.44%) > -NMe2 (2.57%). Electrochemical impedence analysis also confirmed the longer electron life time for dye possessing bulky -NPh2 groups in comparision to -NEt2 and -NMe2 groups, thus demonstrating the impact of increased donor size on the DSSC performance. Overall, this paper validates the impact of donor size on the DSSC performance and could inspire the selection of appropriate auxiliary donors to enhance the efficiency in futuristic endeavors.
An amino-functionalized conjugated metallopolymer PFEN-Hg was developed as a cathode interlayer for inverted polymer solar cells. The resulting devices exhibited significantly improved performance with power conversion efficiencies exceeding 9%. Moreover, good device performance was achievable with the PFEN-Hg over a wider range of film thickness, likely due to the Hg-Hg interactions and improved π-π stacking.
A series of vinylene-linked copolymers based on electron-deficient benzobisthiazole and electron-rich fluorene moieties were synthesized via Horner-Wadsworth-Emmons polymerization. Three different polymers P1, P2, and P3, were prepared bearing octyl, 3,7-dimethyloctyl, and 2-(2-ethoxy)ethoxyethyl side chains, respectively. The polymers all possessed moderate molecular weights, good solubility in aprotic organic solvents, and high fluorescence quantum efficiencies in dilute solutions. P2, which bore branched 3,7-dimethyloctyl side chains, exhibited better solubility than the other polymers, but also exhibited the lowest thermal decomposition temperature of all polymers. Overall, the impact of the side chains on the polymers optical properties in solution was negligible as all three polymers gave similar absorption and emission spectra in both solution and film. Guest-host light-emitting diodes using dilute blends of the polymers in a poly(N-vinylcarbazole) host gave blue-green emission with P2 exhibiting the highest luminous efficiency, 0.61 Cd/A at ~500 nm.
Novel noncovalently connected water-soluble nanoparticles containing hydroxyl-capped polyfluorene (PFOH) and poly-(acrylic acid) (PAA) were obtained and characterized. These nanoparticles were quite stable in water and no precipitate was observed after weeks. Transmission electron microscopy (TEM), scanning electron microscopy (SEM), and laser light scattering (DLS) were used to confirm the morphology of the PFOH/PAA nanoparticles. Their optical properties were investigated and showed the similar optoelectronic properties with PFOH solid film. Copyright
Novel fluorene based deep-blue-emitting molecules with naphthylanthracene endcaps, namely 2,7-di(10-naphthylanthracene-9-yl)-9,9-dioctylfluorene (NAF1) and 7,7′-di(10-naphthylanthracene-9-yl)-9,9,9′,9′-tetraoctyl- 2,2′-bifluorene (NAF2), are synthesized by a Suzuki cross-coupling reaction. These materials exhibit excellent thermal and amorphous stabilities, and high fluorescence quantum yield of over 70%. Organic light-emitting devices (OLEDs) using NAF1 or NAF2 as non-doped emitter exhibit bright deep blue electroluminescence with CIE coordinates of (0.15, 0.13) for NAF1, (0.16, 0.13) for NAF2. A maximum power efficiency of 2.2 lm W-1 (4.04 cd A -1, 4.04%) is achieved for NAF1, which is among the highest values ever reported for deep-blue fluorescent OLEDs. A further improved coordinates of (0.15, 0.09) with efficiencies of 3.56 cd A-1 and 2.10 lm W -1 are achieved for NAF1 upon tuning device thickness, which are also among the best data for non-doped deep blue fluorescent OLEDs with a CIE coordinate of y -1 (7.66 cd A -1), a brightness of 12090 cd m-2, and a standard white light coordinates of (0.33, 0.33). This performance is among the best results ever reported for two-emitting-component white OLEDs based on fluorescent materials.
Star-shaped oligofluorene truxenes are very promising materials and have demonstrated excellent properties as the gain medium in organic semiconductor lasers (OSLs).1-10 Alkyl chains in oligofluorene truxenes act as solubilizing groups as well as spacers to prevent intermolecular π-π stacking that leads to quenching of the light emission. A new series of star-shaped systems analogous to hexyl oligofluorene truxenes11 with alkyl chains of different lengths (butyl chains and octyl chains) was synthesized. The objective of this study was to investigate the effect of alkyl chain length on the film-forming properties of oligofluorene-truxene materials and, as a result, on their optoelectronic properties for applications as the gain medium in OSLs.
AIE/AIEE-active conjugated polymers have shown great potential in bioimaging applications. However, the absorption of many AIE/AIEE polymers poorly matches with the laser excitation used in confocal imaging, which may greatly affect the imaging quality. I
The present application discloses compounds and compositions, useful in the manufacture of dye-sensitized solar cells and other similar technology.
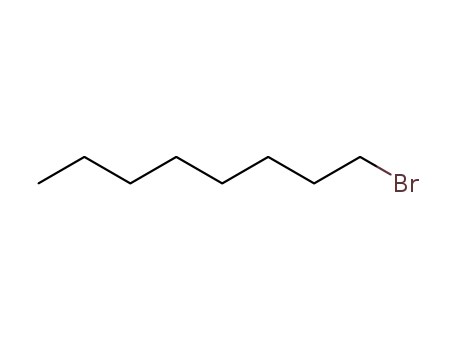
1-bromo-octane

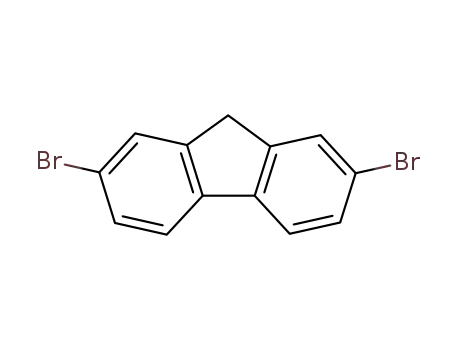
2,7-dibromo-9H-fluorene

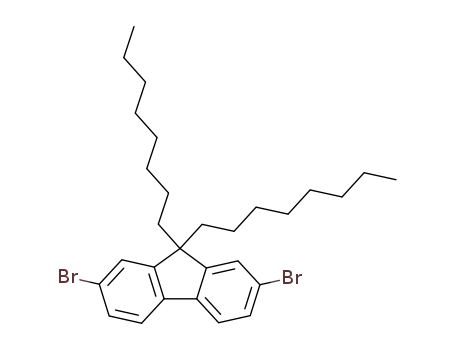
2,7-dibromo-9,9-dioctylfluorene
| Conditions | Yield |
|---|---|
|
With
[benzene-1,3,5-triyltris(methylene)]tris(triphenylphosphonium) tribromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 30 ℃;
for 1h;
Inert atmosphere;
|
96% |
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
91.2% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
toluene;
at 90 ℃;
for 24h;
|
91% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 8h;
|
91% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
toluene;
at 60 ℃;
for 16h;
Inert atmosphere;
|
90% |
|
With
sodium hydroxide;
In
water; toluene;
|
90.2% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 10h;
|
90% |
|
2,7-dibromo-9H-fluorene;
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
1-bromo-octane;
With
3-chloro-benzenecarboperoxoic acid;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
90% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide;
In
dimethyl sulfoxide;
at 20 ℃;
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 0.166667h;
1-bromo-octane;
In
dimethyl sulfoxide;
|
90% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 1h;
Inert atmosphere;
1-bromo-octane;
In
water; dimethyl sulfoxide;
|
90% |
|
With
sodium hydroxide; tetrabutylammomium bromide;
In
toluene;
at 70 ℃;
for 24h;
|
89% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
89% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide; potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 0.5h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 20 ℃;
for 24h;
|
88.6% |
|
With
tetrabutylammomium bromide; potassium hydroxide;
In
acetone;
at 57 ℃;
for 4h;
Reflux;
|
86% |
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
|
85% |
|
|
85% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; toluene;
at 70 ℃;
for 24h;
|
85% |
|
2,7-dibromo-9H-fluorene;
With
potassium iodide; potassium hydroxide;
In
dimethyl sulfoxide;
for 0.166667h;
Cooling with ice;
Inert atmosphere;
1-bromo-octane;
In
dimethyl sulfoxide;
at 20 ℃;
Inert atmosphere;
|
84.1% |
|
2,7-dibromo-9H-fluorene;
With
potassium iodide; potassium hydroxide;
In
dimethyl sulfoxide;
for 0.166667h;
1-bromo-octane;
In
dimethyl sulfoxide;
for 0.5h;
|
84.1% |
|
With
tetrabutylammomium bromide; water; sodium hydroxide;
In
dimethyl sulfoxide;
at 100 ℃;
for 10h;
|
83% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
83% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 100 ℃;
for 10h;
|
83% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide;
In
dimethyl sulfoxide;
for 0.25h;
Inert atmosphere;
1-bromo-octane;
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
Inert atmosphere;
|
83% |
|
With
tert-butylammonium hexafluorophosphate(V); sodium hydroxide;
Inert atmosphere;
|
81% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide; potassium hydroxide;
In
dimethyl sulfoxide;
for 0.5h;
Inert atmosphere;
1-bromo-octane;
In
dimethyl sulfoxide;
at 80 ℃;
for 24h;
Inert atmosphere;
|
80% |
|
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 10.5h;
|
80% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
for 2h;
Inert atmosphere;
1-bromo-octane;
In
water; dimethyl sulfoxide;
for 10h;
|
80% |
|
2,7-dibromo-9H-fluorene;
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
for 2h;
Inert atmosphere;
1-bromo-octane;
In
water; dimethyl sulfoxide;
for 10h;
Inert atmosphere;
|
80% |
|
With
tetra-(n-butyl)ammonium iodide; sodium hydroxide;
In
water; toluene;
at 60 ℃;
for 10h;
Inert atmosphere;
|
80% |
|
With
sodium t-butanolate;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 24h;
|
77% |
|
2,7-dibromo-9H-fluorene;
With
potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 0.166667h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 60 ℃;
for 120h;
|
76% |
|
With
sodium hydroxide; tetrabutylammomium bromide; water;
In
toluene;
for 4h;
|
75% |
|
2,7-dibromo-9H-fluorene;
With
sodium hydride;
In
tetrahydrofuran;
at 60 ℃;
1-bromo-octane;
In
tetrahydrofuran;
Further stages.;
Heating;
|
75.3% |
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; toluene;
at 60 ℃;
for 4h;
Inert atmosphere;
|
75% |
|
2,7-dibromo-9H-fluorene;
With
potassium hydroxide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
1-bromo-octane;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
75% |
|
With
potassium hydroxide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 25h;
|
75% |
|
2,7-dibromo-9H-fluorene;
With
potassium hydroxide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
1-bromo-octane;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
75% |
|
2,7-dibromo-9H-fluorene;
With
potassium hydroxide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
1-bromo-octane;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
75% |
|
2,7-dibromo-9H-fluorene;
With
potassium hydroxide;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 1h;
1-bromo-octane;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
|
75% |
|
With
potassium tert-butylate;
In
tetrahydrofuran;
at 0 - 20 ℃;
for 5.5h;
|
74% |
|
2,7-dibromo-9H-fluorene;
With
potassium iodide; potassium hydroxide;
In
dimethyl sulfoxide;
for 0.5h;
1-bromo-octane;
In
dimethyl sulfoxide;
at 20 ℃;
|
71% |
|
With
sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride;
In
dimethyl sulfoxide;
at 20 ℃;
for 2h;
|
65% |
|
With
sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride;
In
dimethyl sulfoxide;
|
|
|
With
sodium hydroxide; tetrabutylammomium bromide;
In
toluene;
at 60 ℃;
|
|
|
With
sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride;
In
dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
|
|
2,7-dibromo-9H-fluorene;
With
sodium hydroxide;
In
water; dimethyl sulfoxide;
1-bromo-octane;
With
N-benzyl-N,N,N-triethylammonium chloride;
In
water; dimethyl sulfoxide;
|
|
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
|
|
|
With
tetraethylammonium bromide;
In
dimethyl sulfoxide;
|
|
|
With
tetraethylammonium bromide;
In
dimethyl sulfoxide;
|
|
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
toluene;
|
|
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 100 ℃;
for 12h;
|
|
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
water;
Inert atmosphere;
|
|
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
|
|
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
Inert atmosphere;
|
|
|
With
potassium iodide; potassium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
|
|
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
|
|
|
With
sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
|
|
|
2,7-dibromo-9H-fluorene;
With
potassium hydroxide;
In
dimethyl sulfoxide;
for 1h;
Inert atmosphere;
1-bromo-octane;
In
dimethyl sulfoxide;
at 90 ℃;
for 24h;
Inert atmosphere;
|
|
|
2,7-dibromo-9H-fluorene;
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
1-bromo-octane;
In
water; dimethyl sulfoxide;
for 3h;
|
|
|
2,7-dibromo-9H-fluorene;
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
1-bromo-octane;
In
water; dimethyl sulfoxide;
for 3h;
|
|
|
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
for 3h;
|
|
|
With
tetraethylammonium bromide; sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 24h;
Temperature;
Inert atmosphere;
Darkness;
|
|
|
With
N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide;
In
water; dimethyl sulfoxide;
at 20 ℃;
|
|
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
dimethyl sulfoxide;
at 20 ℃;
for 12h;
|
|
|
With
tetra(n-butyl)ammonium hydroxide;
at 80 ℃;
|
|
|
With
tetrabutylammomium bromide; potassium hydroxide;
In
water; dimethyl sulfoxide;
|
|
|
With
potassium hydroxide;
In
dimethyl sulfoxide;
|
|
|
With
tetrabutylammomium bromide; sodium hydroxide;
In
toluene;
|
|
|
With
tetrabutylammomium bromide;
In
N,N-dimethyl-formamide;
at 60 ℃;
for 10h;
|
|
|
With
sodium hydroxide;
In
toluene;
at 60 ℃;
|
9,9-dioctyl-9H-fluorene


2,7-dibromo-9,9-dioctylfluorene
| Conditions | Yield |
|---|---|
|
With
bromine; iron(III) chloride;
In
chloroform;
at 0 - 25 ℃;
for 24h;
|
99% |
|
With
bromine; iron(III) chloride;
In
chloroform;
for 3h;
Ambient temperature;
|
99% |
|
With
benzyltrimethylazanium tribroman-2-uide; zinc(II) chloride;
In
dichloromethane;
at 40 ℃;
for 24h;
Inert atmosphere;
|
98% |
|
With
bromine;
In
N,N-dimethyl-formamide;
Ambient temperature;
|
92% |
|
With
bromine; iron(III) chloride;
In
chloroform;
at 0 - 20 ℃;
for 3h;
|
90% |
|
With
bromine; iron(III) chloride;
In
chloroform;
at 0 - 20 ℃;
|
79% |
|
With
bromine; iodine;
In
dichloromethane;
at 0 - 20 ℃;
for 3h;
|
74% |
|
With
bromine; iron(III) chloride;
|
|
|
With
bromine; iodine;
In
dichloromethane;
at 20 ℃;
for 20h;
|
|
|
With
bromine; iodine;
In
dichloromethane;
at 20 ℃;
|
|
|
With
bromine; iron(III) chloride;
In
chloroform;
at 20 ℃;
for 3h;
|
|
|
With
bromine;
In
chloroform;
at 0 ℃;
|
|
|
With
bromine;
In
N,N-dimethyl-formamide;
|

9,9-dioctyl-9H-fluorene

1-bromo-octane

2,7-dibromo-9H-fluorene
sodium hydroxide
2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9,9-dioctylfluorene
9,9-dioctyl-9H-fluorene-2,7-diyldiboronic acid
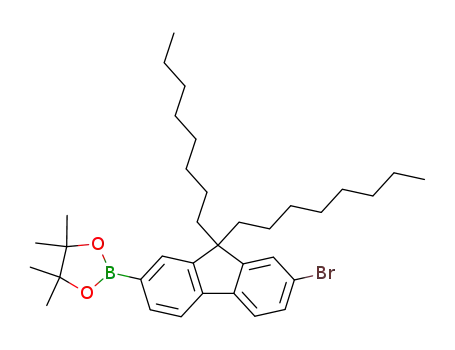
2-(4',4',5',5'-tetramethyl-1',3',2'-dioxaborolane-2'-yl)-7-bromo-9,9-bisoctylfluorene
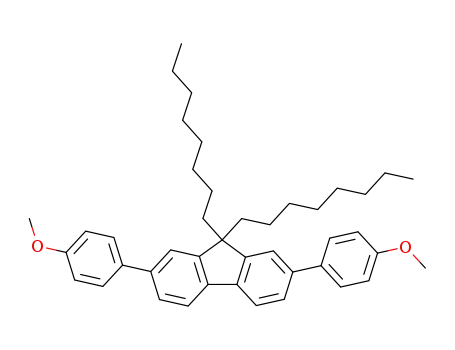
2,7-bis-(4-methoxy-phenyl)-9,9-dioctyl-9H-fluorene
CAS:76189-55-4
Molecular Formula:C44H32P2
Molecular Weight:622.7
CAS:189367-54-2