Your Location:Home >Products >Functional intermediates >76186-72-6

Product Details
Uses
4,7-Dibromo-5,6-dintiro-2,1,3-benzothiadiazole can be incorporated in light-emitting diodes producing near-IR sensitivity.
In this work, the change of reactivity induced by the introduction of two para-ethynyl substituents (CCSi(iPr)3 or CCH) to the organic electron-donor 1,2,4,5-tetrakis(tetramethylguanidino)-benzene is evaluated. The redox-properties and redox-state dependent fluorescence are evaluated, and dinuclear CuI and CuII complexes synthesized. The Lewis-acidic B(C6F5)3 substitutes the proton of the ethynyl ?CCH groups to give new anionic ?CCB(C6F5)3? substituents, leading eventually to a novel dianionic strong electron donor in its diprotonated form. Its two-electron oxidation with dioxygen in the presence of a copper catalyst yields the first redox-active guanidine that is neutral (instead of cationic) in its oxidized form.
Three new fluorinated D-A type conjugated polymers based on a novel building unit 7-perfluorophenyl-6H-[1,2,5]thiadiazole[3,4-g]benzoimidazole have been synthesized through a Sitlle coupling reaction. The as-prepared polymers exhibit a narrow band gap (from 1.31 to 1.34 eV) and low lying energy levels with lowest occupied molecular orbital (LUMO) energy levels of -3.95, -3.97 and -4.15 eV, respectively. These polymers exhibit excellent solubility in common organic solvents due to the introduction of perfluorophenyl and long alkyl sidechains. The power conversion efficiency (PCE) of solar cells based on these as-prepared polymers and PC71BM could reach as high as 1.92%. Our results could provide a simple strategy for designing high performance D-A polymers based on this unit and a potential to further improve their performance.
Most of solid tumor cells are hypoxic and hard to trace and measure. A new compound, 4,7-bis(4-dodecylthiophen-2-yl)-5,6-dinitrobenzo[c][1,2,5]thiadiazole (BTTD-NO2), was synthesized for labeling the hypoxic cells specially in this paper. BTTD-NO2 showed no cytotoxicity to MG63 cells by MTT method. When MG63 cells were cultured with BTTD-NO2 under hypoxic condition for 24 h, strong red fluorescence distribution in cytoplasm was observed. Flow cytometry results showed that 65% of MG63 cells were labeled with strong red fluorescence in hypoxic condition while only 2.4% in oxic condition. Furthermore, Real time RT-PCR proved that BTTD-NO2 could stimulate high gene expression of the nitroreductase in the cells which could improve the conversion rate of BTTD-NO2 to BTTD-NH2 in turn. It proved that the fluorescence of BTTD-NO2 was quenched by its two nitro groups, however, strong red fluorescence could emit in the cytoplasm after the reduction of its nitro groups to amino groups in the tumor cells under hypoxic condition. These results suggested that BTTD-NO2 had the potential as a superior fluorescent probe for tumor detection.
An optimized route toward iptycene-capped, p -dibromo-quinoxalinophenazine was developed, increasing the yield significantly from literature procedures. New iptycene-containing symmetrical aza-acenes were synthesized from this intermediate using Suzuki-Miyaura cross-coupling, and their photophysical properties were evaluated. Tuning the substituents allows modulating emission wavelengths across the visible spectrum. Substitution with 3-methoxy-2-methylthiophene exhibits a quantum yield of 35%. The (triisopropylsilyl)acetylene product has a quantum yield of 38% and serves as a model compound for the synthesis of polymers based on this electrooptically active molecular motif.
-
The invention belongs to the technical field of conjugated polymers, and particularly relates to an ester-substituted aromatic heterocyclic conjugated skeleton and a polymer material and application thereof. The ester-substituted heterocyclic aromatic conjugated skeleton and the polymer material thereof have a larger conjugated plane structure and are more beneficial to intramolecular charge transfer, and compared with the traditional heterocyclic aromatic polymer material, the ester-substituted heterocyclic aromatic conjugated skeleton has the advantages that on the basis of the original chemical structure of the polymer, two strong electron-withdrawing functional groups, namely ester groups, are introduced, so that the aromatic heterocyclic conjugated skeleton has stronger electron-withdrawing capability, the polymer has lower LUMO energy level, redder absorption spectrum and excellent long-wave range light absorption performance, and the ester-substituted aromatic heterocyclic conjugated skeleton and the polymer material thereof are used as photothermal conversion materials to be applied to the field of photoacoustic imaging, and have the advantages of higher photothermal conversion efficiency, better light stability, better imaging effect and the like.
The invention provides a nitration method of a simple and efficient aromatic heterocyclic compound, which is large in width, easy to operate and good in repeatability. By means of the method, high-efficiency nitration of common aromatic compounds is realized, the nitration efficiency is greatly improved, and the simple synthesis of the organic photoelectric material intermediate is realized.
Near-infrared (NIR) small molecular organic dyes as photothermal agents for cancer photothermal therapy (PTT) have attracted considerable research attention. Herein, two donor-acceptor-donor (D-A-D) structured NIR dyes, BBTT and SeBTT, are rationally designed, where the only difference is one heteroatom within the acceptor unit varying from sulfur to selenium (Se). More importantly, SeBTT NPs exhibit stronger NIR absorbance and higher photothermal conversion efficiency (PTCE ≈ 65.3%). In vivo experiments illustrate that SeBTT NPs can be utilized as a high contrast photoacoustic imaging (PAI) agent, and succeed in tumor suppression without noticeable damage to main organs under NIR photoirradiation. This study presents an effective molecular heteroatom surgery strategy to regulate the photothermal properties of NIR small molecules for enhanced PAI and PTT.
Two non-fullerene small molecule acceptors (TFQ-F and TFQ-Cl) based on quinoxaline unit were designed and synthesized for efficient organic solar cells (OSCs). These two acceptors showed intense absorption up to 900 nm and high thermal stabilities with decomposition temperatures over 360 °C due to their fused-ring skeletons. TFQ-F and TFQ-Cl are the A-D-A′-D-A type acceptors (A/A′ for acceptor unit and D for donor unit). TFQ-F and TFQ-Cl have the same D-A′-D fragment, which was flanked with different ending groups. The effect of different ending groups on their photophysical properties, electrochemical behaviors, micro-structures and charge recombination properties of active layers, and device performance were investigated systematically. PM6 with the complementary absorption to the two acceptors was used as the donor material. The pristine PM6:TFQ-F blend films displayed the optimal morphologies as revealed by AFM and TEM measurement. Organic solar cells based on PM6:TFQ-Cl blend film showed high JSC of 25.19 mA/cm2 and PCE of 13.2%. The Voc, JSC and PCE for PM6:TFQ-F film based device were 0.857 V, 23.70 mA/cm2 and 13.51%, respectively. The dependence of VOC/JSC on various light intensities indicated that PM6:TFQ-F/Cl based device had low charge recombination.
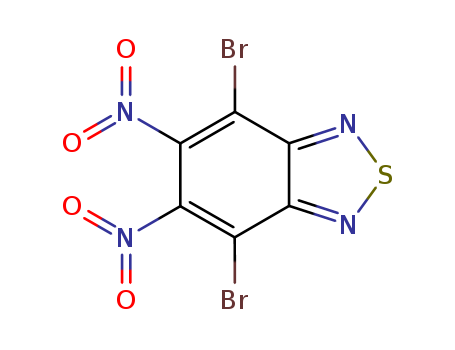
![4,7-dibromobenzo[c][1,2,5]thiadiazole](/upload/2023/2/db6951b7-466d-406c-a9a6-57051dd3af9d.png)
4,7-dibromobenzo[c][1,2,5]thiadiazole

![4,7-dibromo-5,6-dinitrobenzo[2,1,3]thiadiazole](/upload/2023/2/e3dccb60-3464-4b56-9189-9d6e0ae321a3.png)
4,7-dibromo-5,6-dinitrobenzo[2,1,3]thiadiazole
| Conditions | Yield |
|---|---|
|
With
sulfuric acid; nitric acid;
In
toluene;
at 20 ℃;
for 20h;
|
95% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 5 - 50 ℃;
|
94% |
|
With
trifluorormethanesulfonic acid; nitric acid;
|
89% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 0 - 20 ℃;
|
89% |
|
With
sulfuric acid; sulfur trioxide; nitric acid;
at 0 ℃;
for 4h;
|
88% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 50 ℃;
|
85% |
|
With
sulfuric acid; nitric acid;
at 60 ℃;
for 8h;
|
83% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 50 ℃;
|
80% |
|
With
fuming nitric acid;
at 0 - 50 ℃;
for 12h;
|
75% |
|
With
trifluorormethanesulfonic acid; sulfuric acid; nitric acid;
at 40 ℃;
for 13.25h;
Cooling with ice;
|
75% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 0 ℃;
for 24h;
Heating;
|
73% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 0 - 50 ℃;
for 12.5h;
|
73% |
|
With
sulfuric acid; nitric acid;
at 0 ℃;
for 3h;
|
70% |
|
With
trifluorormethanesulfonic acid; nitric acid;
|
67% |
|
With
trifluorormethanesulfonic acid; nitric acid;
Inert atmosphere;
Schlenk technique;
|
67% |
|
With
trifluorormethanesulfonic acid; nitric acid;
|
67% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 0 - 50 ℃;
|
65% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 0 - 50 ℃;
Inert atmosphere;
|
65% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 50 ℃;
for 16.3333h;
Cooling with ice;
|
54% |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 50 ℃;
Cooling with ice;
|
53.4% |
|
With
trifluorormethanesulfonic acid; sulfuric acid; nitric acid;
at 0 - 20 ℃;
|
48% |
|
With
sulfuric acid; nitric acid;
at 0 ℃;
for 6h;
|
40% |
|
With
trifluorormethanesulfonic acid; sulfuric acid; nitric acid;
at 0 - 20 ℃;
for 4h;
|
40% |
|
With
sulfuric acid; nitric acid;
|
36% |
|
With
sulfuric acid; nitric acid;
|
34% |
|
With
sulfuric acid; nitric acid;
at 0 - 20 ℃;
|
33% |
|
With
sulfuric acid; nitric acid;
at 5 - 20 ℃;
|
33% |
|
With
sulfuric acid; nitric acid;
at 10 ℃;
|
33% |
|
With
sulfuric acid; nitric acid;
at 5 - 20 ℃;
|
33% |
|
With
sulfuric acid; nitric acid;
at 3 - 20 ℃;
|
31% |
|
With
sulfuric acid; nitric acid;
at 3 - 20 ℃;
|
31% |
|
With
sulfuric acid; nitric acid;
|
28% |
|
With
sulfuric acid; nitric acid;
at 0 ℃;
for 6h;
|
27% |
|
With
sulfuric acid; nitric acid;
at 3 - 20 ℃;
|
26% |
|
With
sulfuric acid; nitric acid;
|
25.5% |
|
With
sulfuric acid; nitric acid;
1) 0 to 5 deg C, 30 min, 2) RT, 2 h;
|
20% |
|
With
nitric acid; trifluoroacetic acid;
In
dichloromethane;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
at 5 - 25 ℃;
for 21h;
|
|
|
With
sulfuric acid; nitric acid;
|
|
|
With
sulfuric acid; nitric acid;
at 5 - 25 ℃;
for 21h;
|
60 g |
|
With
sulfuric acid; nitric acid;
at 5 - 25 ℃;
for 21h;
|
60 g |
|
With
sulfuric acid; nitric acid;
In
toluene;
at 25 ℃;
for 21h;
|
60 g |
|
With
sulfuric acid; nitric acid;
at 5 - 25 ℃;
for 21h;
|
|
|
With
sulfuric acid; nitric acid;
at 5 - 50 ℃;
for 21h;
|
60 g |
|
With
trifluorormethanesulfonic acid; nitric acid;
at 0 - 50 ℃;
|
|
|
With
trifluorormethanesulfonic acid; nitric acid;
Inert atmosphere;
|
|
|
With
trifluorormethanesulfonic acid; nitric acid;
Inert atmosphere;
|
|
|
With
nitric acid; trifluoroacetic acid;
|
|
|
With
trifluorormethanesulfonic acid; nitric acid;
at 20 - 50 ℃;
|
![benzo[1,2,5]thiadiazole](/upload/2023/2/47e154d7-450e-449a-80f4-9d3a5667c798.png)
benzo[1,2,5]thiadiazole

![4,7-dibromo-5,6-dinitrobenzo[2,1,3]thiadiazole](/upload/2023/2/e3dccb60-3464-4b56-9189-9d6e0ae321a3.png)
4,7-dibromo-5,6-dinitrobenzo[2,1,3]thiadiazole
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: aq. HBr; Br2 / 130 °C
2: CF3CO2H; HNO3 / CH2Cl2
With
hydrogen bromide; bromine; nitric acid; trifluoroacetic acid;
In
dichloromethane;
|
|
|
Multi-step reaction with 2 steps
1: hydrogen bromide; bromine
2: trifluorormethanesulfonic acid; nitric acid
With
trifluorormethanesulfonic acid; hydrogen bromide; bromine; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: hydrogen bromide; bromine / water
2: nitric acid; sulfuric acid
With
sulfuric acid; hydrogen bromide; bromine; nitric acid;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: bromine; hydrogen bromide
2: nitric acid; sulfuric acid
With
sulfuric acid; hydrogen bromide; bromine; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: bromine; hydrogen bromide / water / Reflux
2: nitric acid; trifluorormethanesulfonic acid; sulfuric acid / 4 h / 0 - 20 °C
With
trifluorormethanesulfonic acid; sulfuric acid; hydrogen bromide; bromine; nitric acid;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: bromine; hydrogen bromide / water / 6 h / 100 °C
2: nitric acid; sulfuric acid / 0 - 20 °C
With
sulfuric acid; hydrogen bromide; bromine; nitric acid;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: bromine; hydrogen bromide / water / 125 - 130 °C
2: nitric acid; trifluorormethanesulfonic acid / 5 - 50 °C
With
trifluorormethanesulfonic acid; hydrogen bromide; bromine; nitric acid;
In
water;
|
|
|
Multi-step reaction with 2 steps
1: hydrogen bromide; bromine / 6 h / 110 °C
2: nitric acid; sulfuric acid / 3 h / 0 °C
With
sulfuric acid; hydrogen bromide; bromine; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: hydrogen bromide; bromine / 6 h / Reflux
2: nitric acid; trifluorormethanesulfonic acid / 24 h / 0 °C / Heating
With
trifluorormethanesulfonic acid; hydrogen bromide; bromine; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: hydrogen bromide; bromine / 6 h / Reflux
2: nitric acid; trifluorormethanesulfonic acid / 0 - 50 °C
With
trifluorormethanesulfonic acid; hydrogen bromide; bromine; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: bromine; hydrogen bromide / Inert atmosphere
2: nitric acid; trifluorormethanesulfonic acid / Inert atmosphere
With
trifluorormethanesulfonic acid; hydrogen bromide; bromine; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: N-Bromosuccinimide; sulfuric acid / 3 h / 60 °C
2: trifluorormethanesulfonic acid; nitric acid / 20 - 50 °C
With
N-Bromosuccinimide; trifluorormethanesulfonic acid; sulfuric acid; nitric acid;
|
|
|
Multi-step reaction with 2 steps
1: bromine; hydrogen bromide / 6 h / Reflux
2: sulfuric acid; nitric acid / 8 h / 60 °C
With
sulfuric acid; hydrogen bromide; bromine; nitric acid;
|
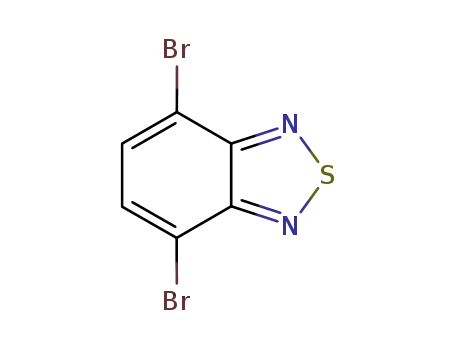
4,7-dibromobenzo[c][1,2,5]thiadiazole
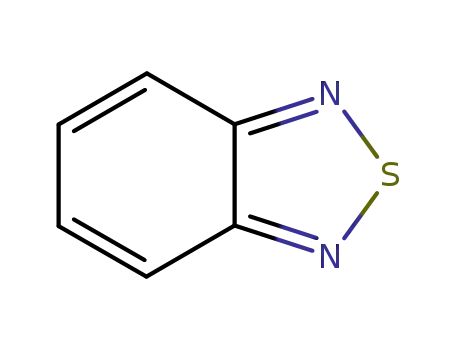
benzo[1,2,5]thiadiazole
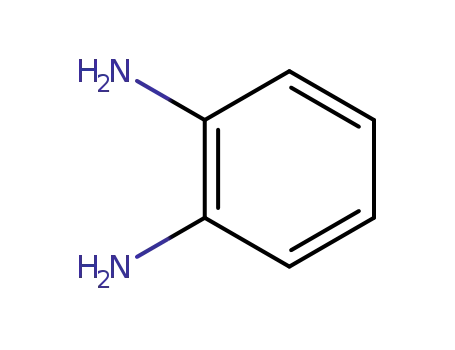
1,2-diamino-benzene
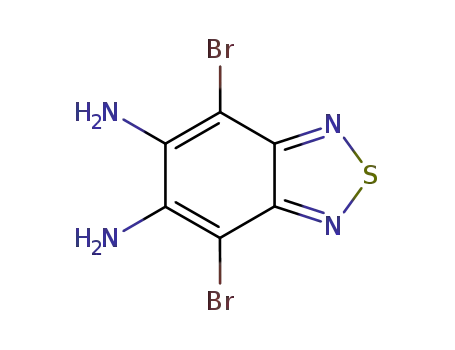
5,6-diamino-4,7-dibromobenzo[c][1,2,5]-thiadiazole
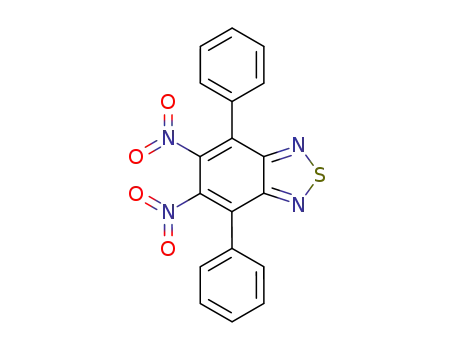
5,6-dinitro-4,7-diphenylbenzo[c][1,2,5]thiadiazole
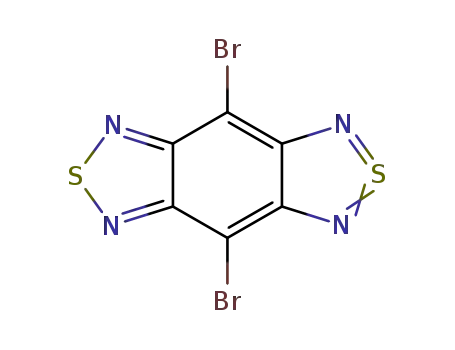
4,8-dibromo-1H,5H-benzo[1,2-c:4,5-c']bis([1,2,5] thiadiazole)
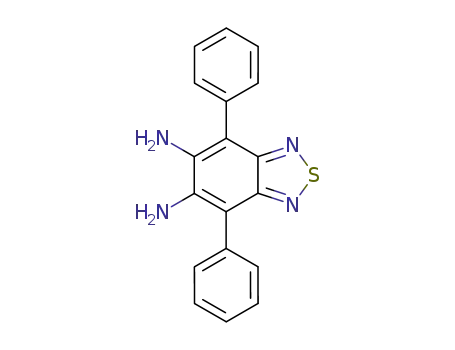
4,7-diphenylbenzo[1,2,5]thiadiazolo-5,6-diamine
CAS:1028647-93-9
Molecular Formula:C24H16BrN
Molecular Weight:398.3
CAS:1055888-89-5
CAS:626-56-2
CAS:7293-45-0