Your Location:Home >Products >Functional intermediates >626-56-2

Product Details
Chemical Properties
clear colorless to yellow liquid
Uses
3-Methylpiperidine, is used as a reactant for synthesis of Phenylpropenamide derivatives for anti-hepatitis B virus activity, CB2 receptor agonists for treatment of chronic pain, aurora kinase inhibitors, spiroimidazolidinone NPC1L1 inhibitors, 1,3,5-Oxadiazinones, selective serotonin 5-HT6 receptor antagonists. It is also used as a pharmaceutical intermediate and as a intermediate in organic synthesis.
General Description
A colorless liquid with a characteristic odor. Less dense than water. Flash point less than 141°F. Contact may cause severe irritation to skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
3-METHYLPIPERIDINE neutralizes acids to form salts plus water in exothermic reactions. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Generates flammable gaseous hydrogen in combination with strong reducing agents, such as hydrides.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Purification Methods
Purify it via the hydrochloride (m 172o). The hydrobromide has m 162-163o(from iso-PrOH). [Chapman et al. J Chem Soc 1925 1959, Beilstein 20 III/IV 1499, 20/4 V 100.]
InChI:InChI=1/C6H13N/c1-6-3-2-4-7-5-6/h6-7H,2-5H2,1H3/p+1/t6-/m0/s1
The invention discloses a method for preparing a piperazine compound through a hydrogen transfer reduction of a pyridine compound, belonging to the field of organic synthesis. Under mild conditions, pyridine derivatives are used as raw materials, oxazolidine is used as a hydrogen transfer reagent, and cheap transition metals such as copper, cobalt, silver, palladium and the like are used as catalysts for catalysis of a hydrogen transfer reaction on 1,2,3,4-substitution sites, so a series of hydrogen transfer reduction product piperidine compounds are prepared, wherein the oxazaborolidine is obtained by a reaction of amino acid with a tetrahydrofuran complex of borane. The method has the advantages that product yield is high, reaction conditions are mild, the general applicability of raw materials is good, a hydrogen transfer reagent is cheap and easy to obtain, and good reproducibility can still be shown after quantitative reaction is conudcted. Therefore, the method of the invention provides an effective scheme for the industrial production of other high-value compounds containing the structure in the future.
Selective hydrogenation of aromatic amines, especially chemicals such as aniline and bis(4-aminocyclohexyl)methane for non-yellowing polyurethane, is of particular interests due to the extensive applications. To conquer the existing difficulties in selective hydrogenation, the Ru0-Ruδ+/CeO2 catalyst with solid frustrated Lewis pairs was developed for aromatic amines hydrogenation with excellent activity and selectivity under relative milder conditions. The morphology, electronic and chemical properties, especially the Ru0-Ruδ+ clusters and reducible ceria were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electronic microscopy (SEM), X-ray photoelectron spectroscopy (XPS), CO2 temperature programmed desorption (CO2-TPD), H2 temperature programmed reduction (H2-TPR), H2 diffuse reflectance Fourier transform infrared spectroscopy (H2-DRIFT), Raman, etc. The 2% Ru/CeO2 catalyst exhibited good conversion of 95% and selectivity greater than 99% toward cyclohexylamine. The volcano curve describing the activity and Ru state was found. Owning to the “acidic site isolation” by surrounding alkaline sites, condensation between the neighboring amine molecules could be effectively suppressed. The catalyst also showed good stability and applicability for other aromatic amines and heteroarenes containing different functional groups.
We have developed a scalable platform that employs electrolysis for an in vitro synthetic enzymatic cascade in a continuous flow reactor. Both H2 and O2 were produced by electrolysis and transferred through a gas-permeable membrane into the flow system. The membrane enabled the separation of the electrolyte from the biocatalysts in the flow system, where H2 and O2 served as electron mediators for the biocatalysts. We demonstrate the production of methylated N-heterocycles from diamines with up to 99 percent product formation as well as excellent regioselective labeling with stable isotopes. Our platform can be applied for a broad panel of oxidoreductases to exploit electrical energy for the synthesis of fine chemicals.
A rhodium-catalyzed method for the hydrogenation of N-heteroarenes is described. A diverse array of unsubstituted N-heteroarenes including pyridine, pyrrole, and pyrazine, traditionally challenging substrates for hydrogenation, were successfully hydrogenated using the organometallic precatalysts, [(η5-C5Me5)Rh(N-C)H] (N-C = 2-phenylpyridinyl (ppy) or benzo[h]quinolinyl (bq)). In addition, the hydrogenation of polyaromatic N-heteroarenes exhibited uncommon chemoselectivity. Studies into catalyst activation revealed that photochemical or thermal activation of [(η5-C5Me5)Rh(bq)H] induced C(sp2)-H reductive elimination and generated the bimetallic complex, [(η5-C5Me5)Rh(μ2,η2-bq)Rh(η5-C5Me5)H]. In the presence of H2, both of the [(η5-C5Me5)Rh(N-C)H] precursors and [(η5-C5Me5)Rh(μ2,η2-bq)Rh(η5-C5Me5)H] converted to a pentametallic rhodium hydride cluster, [(η5-C5Me5)4Rh5H7], the structure of which was established by NMR spectroscopy, X-ray diffraction, and neutron diffraction. Kinetic studies on pyridine hydrogenation were conducted with each of the isolated rhodium complexes to identify catalytically relevant species. The data are most consistent with hydrogenation catalysis prompted by an unobserved multimetallic cluster with formation of [(η5-C5Me5)4Rh5H7] serving as a deactivation pathway.
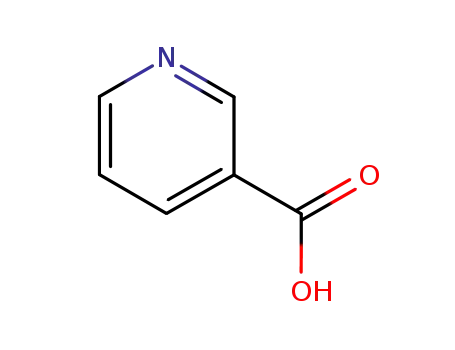
nicotinic acid

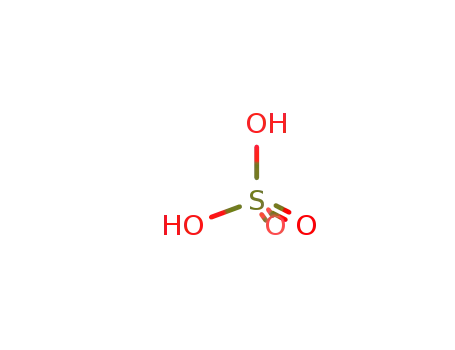
sulfuric acid

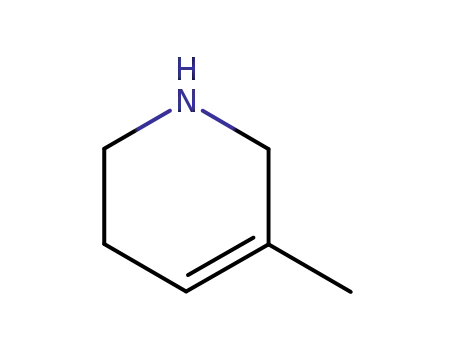
5-methyl-1,2,3,6-tetrahydropyridine

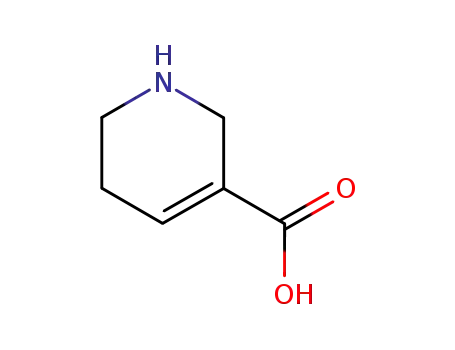
guvacine

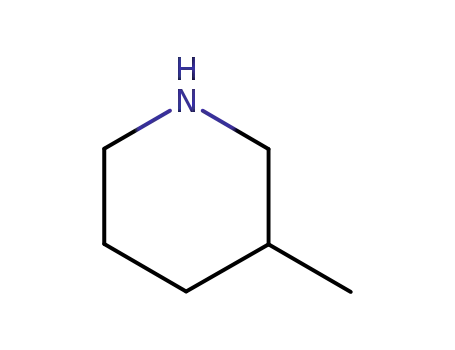
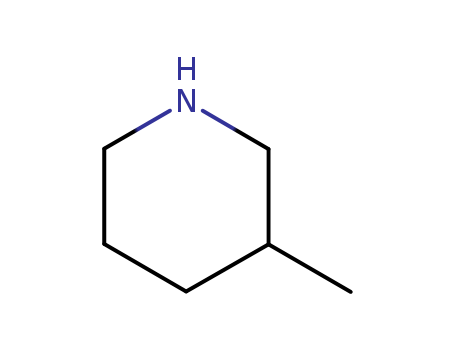
3-Methylpiperidine
| Conditions | Yield |
|---|---|
|
Elektrochemische Reduktion;
|
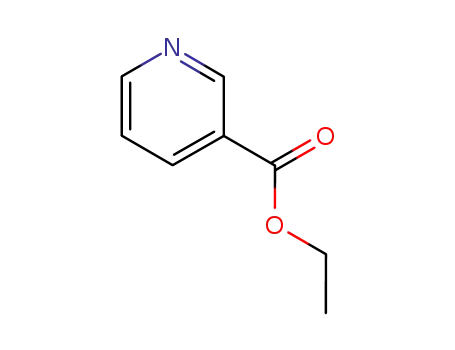
3-pyridinecarboxylic acid ethyl ester


sulfuric acid


5-methyl-1,2,3,6-tetrahydropyridine

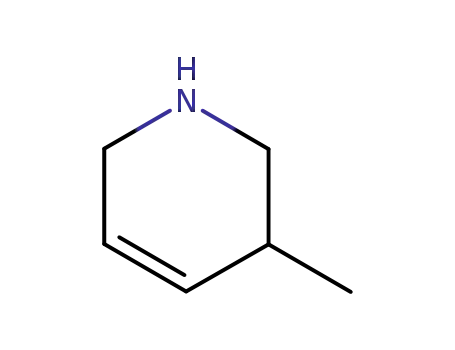
3-methyl-1,2,3,6-tetrahydro-pyridine


guvacine


3-Methylpiperidine
| Conditions | Yield |
|---|---|
|
Elektrochemische Reduktion;
|
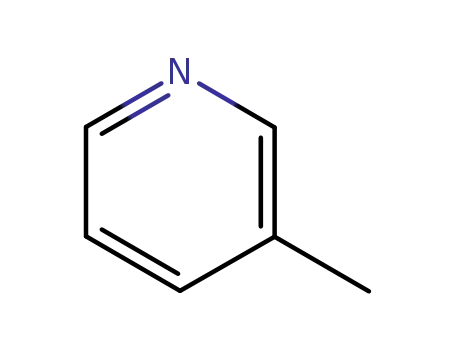
3-Methylpyridine
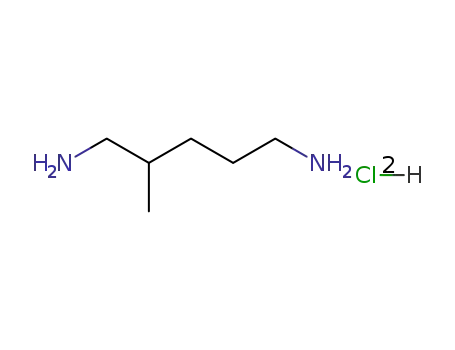
2-methylcadaverine dihydrochloride
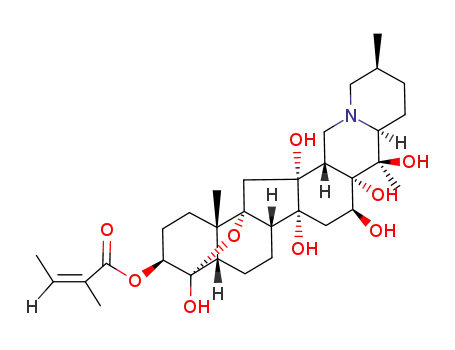
veratrine
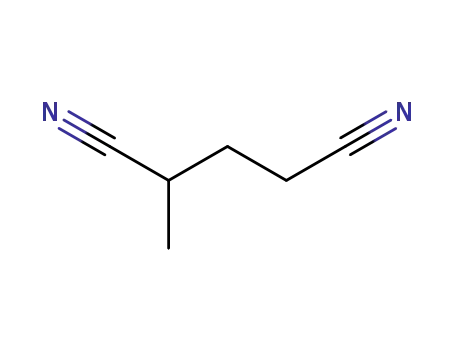
2-methylglutaronitrile
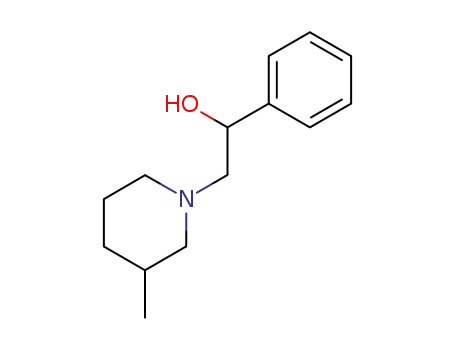
2-(3-methyl-piperidin-1-yl)-1-phenyl-ethanol

3-Methylpyridine
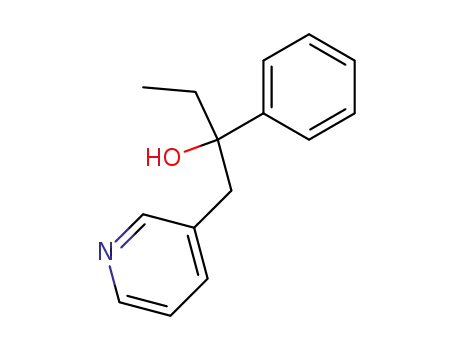
2-phenyl-1-[3]pyridyl-butan-2-ol
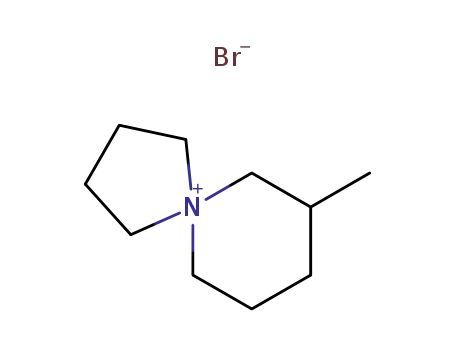
7-methyl-5-azoniaspiro[4,5]decane bromide
CAS:57103-20-5
CAS:1450933-18-2
Molecular Formula:C25H15Br
Molecular Weight:395.3
CAS:76186-72-6