Your Location:Home >Products >Functional intermediates >1221821-41-5

Product Details
A metal-free organic sensitizer, suitable for the application in dye-sensitized solar cells (DSSCs), has been designed, synthesized and characterized both experimentally and theoretically. The structure of the novel donor–acceptor–π-bridge–acceptor (D–A–π–A) dye incorporates a triphenylamine (TPA) segment and 4-(benzo[c][1,2,5]thiadiazol-4-ylethynyl)benzoic acid (BTEBA). The triphenylamine unit is widely used as an electron donor for photosensitizers, owing to its nonplanar molecular configuration and excellent electron-donating capability, whereas 4-(benzo[c][1,2,5]thiadiazol-4-ylethynyl)benzoic acid is used as an electron acceptor unit. The influences of I3?/I?, [Co(bpy)3]3+/2+ and [Cu(tmby)2]2+/+ (tmby=4,4′,6,6′-tetramethyl-2,2′-bipyridine) as redox electrolytes on the DSSC device performance were also investigated. The maximal monochromatic incident photon-to-current conversion efficiency (IPCE) reached 81 % and the solar light to electrical energy conversion efficiency of devices with [Cu(tmby)2]2+/+ reached 7.15 %. The devices with [Co(bpy)3]3+/2+ and I3?/I? electrolytes gave efficiencies of 5.22 % and 6.14 %, respectively. The lowest device performance with a [Co(bpy)3]3+/2+-based electrolyte is attributed to increased charge recombination.
Three new triphenylamine-BODIPY dyadsBDPT1-3have been designed and synthesized. Their optoelectronic properties were investigated, which revealed strong electronic interactions between the donor and acceptor moieties, together with high sensitivity to the inclusion of alkoxy groups. The properties of the dyads were compared with those of reference compoundsAandBDP1, which exhibit broader absorption in the visible region as a result of the inclusion of donor groups and extended conjugation of the BODIPY core. Fluorescence quenching was also observed, which was attributed to the photoinduced electron transfer, evidenced from solvatochromic measurements, quantum yields and theoretical calculations. The oxidation potentials of new compounds were found to be lower when compared with those of other BODIPY analogues with donor groups attached. The redox, computational, absorbance and emission data suggest that compoundsBDPT1-3exhibit promising properties for their application in organic photovoltaic or light emitting (optoelectronic) devices.
A synthetic sequence for the preparation of fully conjugated 2,7-disubstituted fluorazone (9H-pyrrolo[1,2--a]indol-9-one) derivatives has been developed comprising an Elming–Clauson–Kaas-type pyrrole formation, POCl3-mediated ring closure, selective halogenation and elongation of the conjugated backbone through cross-coupling reactions. As a proof of principle, this methodology was used to prepare for the first time two organic D–π–A dyes containing the fluorazone moiety. The new compounds display broad absorption bands in the visible-light region when adsorbed on nanocrystalline TiO2 and electrochemical properties compatible with their employment as photosensitizers in dye-sensitized solar cells. Small-scale photovoltaic devices fabricated with the fluorazone dyes yielded power conversion efficiencies in the range of 2.1–2.4 %, which correspond to approximately 70 % of the efficiency obtained with the reference organic dye DF15 under the same conditions.
A new D-π-A organic dye, LC-5, containing 4,9-dihydro-4,4,9,9- tetrahexyl-s-indaceno[1,2-b:5,6-b′]-dithiophene as a novel π-conjugated spacer has been synthesized and tested as a sensitizer in dye-sensitized solar cells (DSC). Volatile and ionic liquid electrolytes have been used in conjunction with the synthesized dye, and the electrolyte influence on the photovoltaic performance of DSCs was investigated. A detailed investigation, including transient photocurrent/photovoltage decay measurements and electrochemical impedance spectroscopy data, provide important conclusions about the influence of electrolytes on the photovoltaic parameters.
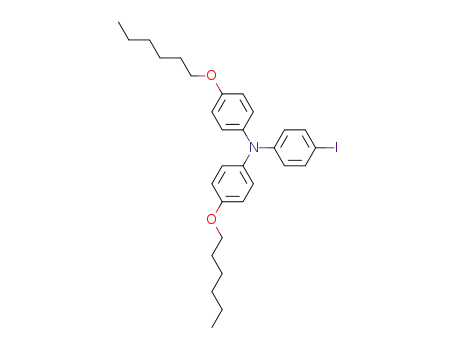
N,N-bis(4-hexyloxyphenyl)-N-(4-iodophenyl) amine

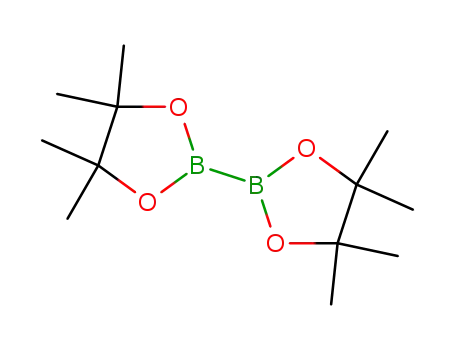
bis(pinacol)diborane


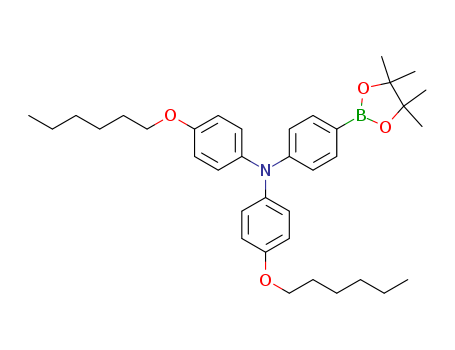
4-(hexyloxy)-N-(4-(hexyloxy)phenyl)-N-(4-(4,4,5,5,-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline
| Conditions | Yield |
|---|---|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
dimethyl sulfoxide;
at 20 ℃;
for 48h;
Inert atmosphere;
|
81% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; dimethyl sulfoxide;
at 80 ℃;
for 5.5h;
Glovebox;
Inert atmosphere;
|
72% |
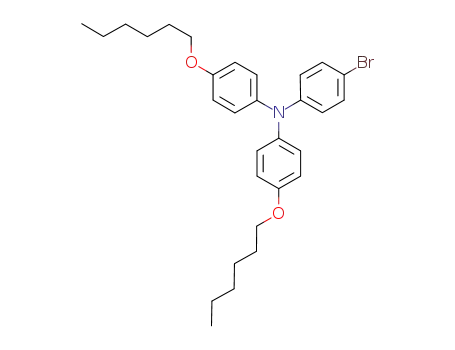
N,N-bis(4-hexyloxyphenyl)-4-bromophenylamine


bis(pinacol)diborane


4-(hexyloxy)-N-(4-(hexyloxy)phenyl)-N-(4-(4,4,5,5,-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline
| Conditions | Yield |
|---|---|
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
1,4-dioxane;
at 100 ℃;
for 20h;
Inert atmosphere;
|
82.9% |
|
With
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate;
In
N,N-dimethyl-formamide;
at 80 ℃;
for 16h;
Schlenk technique;
|
63% |
|
With
tris-(dibenzylideneacetone)dipalladium(0); potassium acetate; XPhos;
In
1,4-dioxane;
at 80 ℃;
for 12h;
Inert atmosphere;
|
48% |

bis(pinacol)diborane
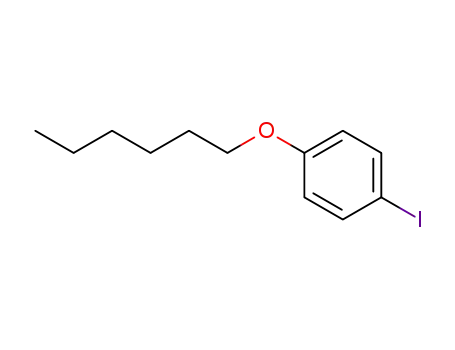
1-iodo-4-hexyloxybenzene
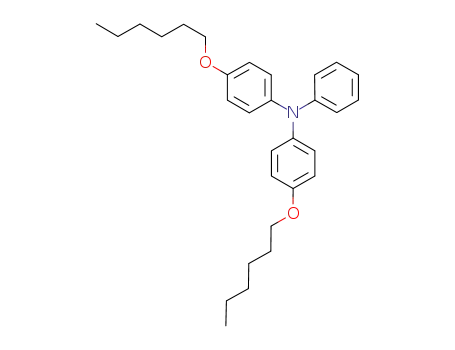
4-(hexyloxy)-N-(4-(hexyloxy)phenyl)-N-phenylaniline
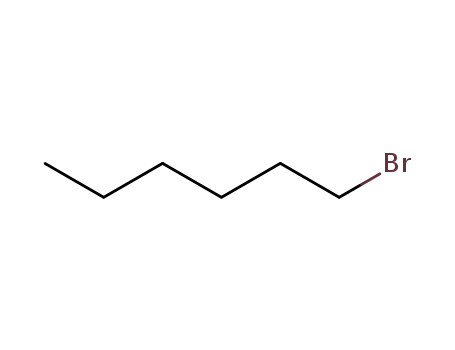
1-bromo-hexane
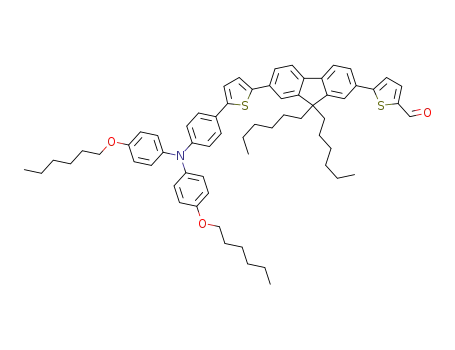
5-(7-(5-(4-(bis(4-(hexyloxy)phenyl)amino)phenyl)thiophen-2-yl)-9,9-dihexyl-9H-fluoren-2-yl)thiophene-2-carbaldehyde
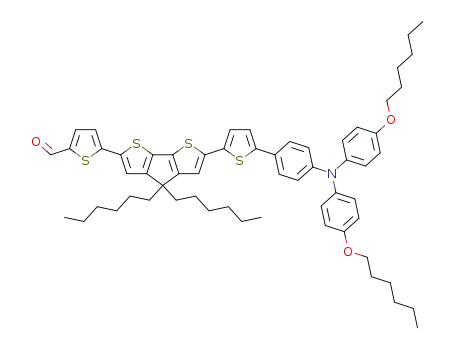
5-(6-(5-(4-(bis(4-(hexyloxy)phenyl)amino)phenyl)thiophen-2-yl)-4,4-dihexyl-4H-cyclopenta[2,1-b:3,4-b']dithiophen-2-yl)thiophene-2-carbaldehyde
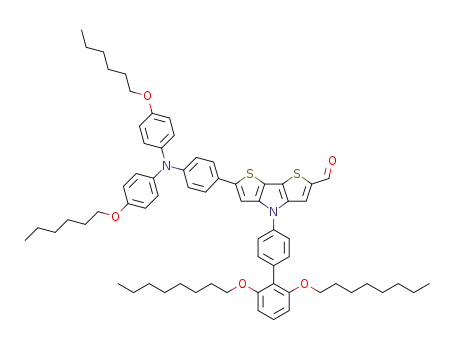
6-{4-[N,N-bis(4-hexyloxyphenyl)amino]phenyl}-4-(2',6'-bis(octyloxy)biphenyl-4-yl)-4H-dithieno[3,2-b:2',3'-d]pyrrole-2-carbaldehyde
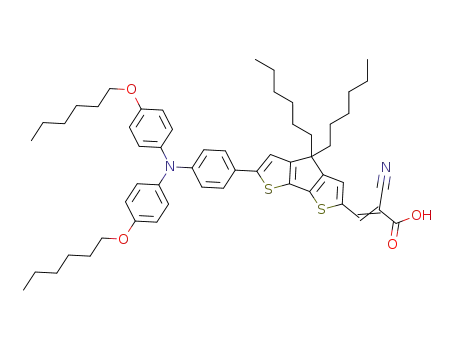
2-cyano-3-{6-{4-[N,N-bis(4-hexyloxyphenyl)amino]phenyl}-4,4-dihexyl-4H-cyclopenta[2,1-b:3,4-b']dithiophene-2-yl}acrylic acid
CAS:5315-79-7
CAS:18666-26-7
CAS:728039-63-2